Propagation of pathology through brain networks in neurodegenerative diseases: from molecules to clinical phenotypes
- PMID: 26031656
- PMCID: PMC6493163
- DOI: 10.1111/cns.12410
Propagation of pathology through brain networks in neurodegenerative diseases: from molecules to clinical phenotypes
Abstract
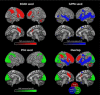
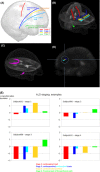
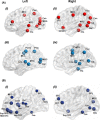
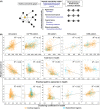
The cellular mechanisms underlying the stereotypical progression of pathology in neurodegenerative diseases are incompletely understood, but increasing evidence indicates that misfolded protein aggregates can spread by a self-perpetuating neuron-to-neuron transmission. Novel neuroimaging techniques can help elucidating how these disorders spread across brain networks. Recent knowledge from structural and functional connectivity studies suggests that the relation between neurodegenerative diseases and distinct brain networks is likely to be a strict consequence of diffuse network dynamics. Diffusion tensor magnetic resonance imaging also showed that measurement of white matter tract involvement can be a valid surrogate to assess the in vivo spreading of pathological proteins in these conditions. This review will introduce briefly the main molecular and pathological substrates of the most frequent neurodegenerative diseases and provide a comprehensive overview of neuroimaging findings that support the "network-based neurodegeneration" hypothesis in these disorders. Characterizing network breakdown in neurodegenerative diseases will help anticipate and perhaps prevent the devastating impact of these conditions.
Keywords: Diffusion tensor MRI; Network-based neurodegeneration; Neurodegenerative diseases; Prion-like proteins; Resting-state functional MRI.
© 2015 John Wiley & Sons Ltd.
Conflict of interest statement
F. Agosta serves on the editorial board of the Journal of Neurology; has received speaker honoraria from Biogen Idec and EXCEMED—Excellence in Medical Education; and receives research supports from the Italian Ministry of Health, and AriSLA (Fondazione Italiana di Ricerca per la SLA). M. Weiler reports no disclosures. M. Filippi is Editor‐in‐Chief of the Journal of Neurology; serves on scientific advisory boards for Teva Pharmaceutical Industries; has received compensation for consulting services and/or speaking activities from Bayer Schering Pharma, Biogen Idec, Merck Serono, Novartis, and Teva Pharmaceutical Industries; and receives research support from Bayer Schering Pharma, Biogen Idec, Merck Serono, Teva Pharmaceutical Industries, Novartis, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, Cure PSP, Alzheimer's Drug Discovery Foundation (ADDF), the Jacques and Gloria Gossweiler Foundation (Switzerland), and AriSLA (Fondazione Italiana di Ricerca per la SLA).
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

