Symptom Control Trials in Patients With Advanced Cancer: A Qualitative Study
- PMID: 26031710
- PMCID: PMC4627489
- DOI: 10.1016/j.jpainsymman.2015.05.009
Symptom Control Trials in Patients With Advanced Cancer: A Qualitative Study
Abstract
Context: Symptom control research in patients with advanced cancer is not common. This may be the result of a belief that this research is unethical, not practical, or that patients are not interested. However, the experiences of cancer patients who have actually taken part in symptom control research near the end of life have never been detailed.
Objectives: The objective was to explore the experiences of patients with advanced cancer who had taken part in symptom control trials.
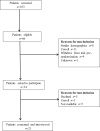
Methods: A prospective two-center study was undertaken using grounded theory methodology. Theoretical sampling was used to recruit patients from one of two double-blind, randomized, placebo-controlled trials studying novel analgesic agents for cancer-related pain. Participants completed one semistructured interview. Recruitment and interviewing continued until data saturation was achieved.
Results: Twenty-one participants were recruited. Fifteen (71%) were male, with a mean age of 62 years. Key themes identified included reasons for trial participation, participants' interactions with the trial staff, and participants' responses to the effect the trial had on their pain. In general, participants regarded taking part in a clinical trial as a positive experience, and potentially improving overall well-being. Crucially, this was not related to whether there had been an improvement in symptoms.
Conclusion: The findings provide grounds for optimism that patients with advanced cancer may benefit from taking part in symptom control trials, supporting the paradigm that participation in symptom control research should be encouraged in this population.
Keywords: Palliative care; cancer; research.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Teunissen S.C., Wesker W., Kruitwagen C. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94–104. - PubMed
-
- Abernethy A.P., Hanson L.C., Main D.S., Kutner J.S. Palliative care clinical research networks, a requirement for evidence-based palliative care: time for coordinated action. J Palliat Med. 2007;10:845–850. - PubMed
-
- Lunder U., Sauter S., Furst C.J. Evidence-based palliative care: beliefs and evidence for changing practice. Palliat Med. 2004;18:265–266. - PubMed
-
- Fainsinger R. Global warming in the palliative care research environment: adapting to change. Palliat Med. 2008;22:328–335. - PubMed
-
- Fine P.G. Maximizing benefits and minimizing risks in palliative care research that involves patients near the end of life. J Pain Symptom Manage. 2003;25:S53–S62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

