Interleukin-10 plays a key role in the modulation of neutrophils recruitment and lung inflammation during infection by Streptococcus pneumoniae
- PMID: 26032199
- PMCID: PMC4552505
- DOI: 10.1111/imm.12486
Interleukin-10 plays a key role in the modulation of neutrophils recruitment and lung inflammation during infection by Streptococcus pneumoniae
Abstract
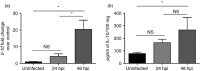
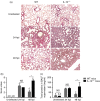
Streptococcus pneumoniae is a major aetiological agent of pneumonia worldwide, as well as otitis media, sinusitis, meningitis and sepsis. Recent reports have suggested that inflammation of lungs due to S. pneumoniae infection promotes bacterial dissemination and severe disease. However, the contribution of anti-inflammatory molecules to the pathogenesis of S. pneumoniae remains unknown. To elucidate whether the production of the anti-inflammatory cytokine interleukin-10 (IL-10) is beneficial or detrimental for the host during pneumococcal pneumonia, we performed S. pneumoniae infections in mice lacking IL-10 (IL-10(-/-) mice). The IL-10(-/-) mice showed increased mortality, higher expression of pro-inflammatory cytokines, and an exacerbated recruitment of neutrophils into the lungs after S. pneumoniae infection. However, IL-10(-/-) mice showed significantly lower bacterial loads in lungs, spleen, brain and blood, when compared with mice that produced this cytokine. Our results support the notion that production of IL-10 during S. pneumoniae infection modulates the expression of pro-inflammatory cytokines and the infiltration of neutrophils into the lungs. This feature of IL-10 is important to avoid excessive inflammation of tissues and to improve host survival, even though bacterial dissemination is less efficient in the absence of this cytokine.
Keywords: bacterial infection; cytokines; lung inflammation; neutrophils; systemic bacterial infection.
© 2015 John Wiley & Sons Ltd.
Figures





References
-
- Nieto PA, Riquelme SA, Riedel CA, Kalergis AM, Bueno SM. Gene elements that regulate Streptococcus pneumoniae virulence and immunity evasion. Curr Gene Ther. 2013;13:51–64. - PubMed
-
- Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6:288–301. - PubMed
-
- O’Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. - PubMed
-
- Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20(Suppl. 5):45–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

