One Crystal, Two Temperatures: Cryocooling Penalties Alter Ligand Binding to Transient Protein Sites
- PMID: 26032594
- PMCID: PMC4539595
- DOI: 10.1002/cbic.201500196
One Crystal, Two Temperatures: Cryocooling Penalties Alter Ligand Binding to Transient Protein Sites
Abstract
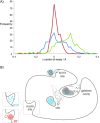
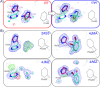
Interrogating fragment libraries by X-ray crystallography is a powerful strategy for discovering allosteric ligands for protein targets. Cryocooling of crystals should theoretically increase the fraction of occupied binding sites and decrease radiation damage. However, it might also perturb protein conformations that can be accessed at room temperature. Using data from crystals measured consecutively at room temperature and at cryogenic temperature, we found that transient binding sites could be abolished at the cryogenic temperatures employed by standard approaches. Changing the temperature at which the crystallographic data was collected could provide a deliberate perturbation to the equilibrium of protein conformations and help to visualize hidden sites with great potential to allosterically modulate protein function.
Keywords: X-ray diffraction; allosterism; biophysics; ligand discovery; structural biology; thermodynamics.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures