The Akt inhibitor, triciribine, ameliorates chronic hypoxia-induced vascular pruning and TGFβ-induced pulmonary fibrosis
- PMID: 26033700
- PMCID: PMC4543621
- DOI: 10.1111/bph.13203
The Akt inhibitor, triciribine, ameliorates chronic hypoxia-induced vascular pruning and TGFβ-induced pulmonary fibrosis
Abstract
Background and purpose: Interstitial lung disease accounts for a group of chronic and progressive disorders associated with severe pulmonary vascular remodelling, peripheral vascular rarefaction and fibrosis, thus limiting lung function. We have previously shown that Akt is necessary for myofibroblast differentiation, a critical event in organ fibrosis. However, the contributory role of the Akt-mTOR pathway in interstitial lung disease and the therapeutic benefits of targeting Akt and mTOR remain unclear.
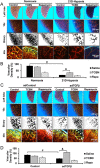
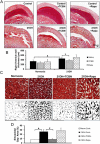
Experimental approach: We investigated the role of the Akt-mTOR pathway and its downstream molecular mechanisms in chronic hypoxia- and TGFβ-induced pulmonary vascular pruning and fibrosis in mice. We also determined the therapeutic benefits of the Akt inhibitor triciribine and the mTOR inhibitor rapamycin for the treatment of pulmonary fibrosis in mice.
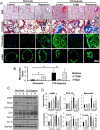
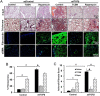
Key results: Akt1(-) (/) (-) mice were protected from chronic hypoxia-induced peripheral vascular pruning. In contrast, hyperactivation of Akt1 induced focal fibrosis similar to TGFβ-induced fibrosis. Pharmacological inhibition of Akt, but not the Akt substrate mTOR, inhibited hypoxia- and TGFβ-induced pulmonary vascular rarefaction and fibrosis. Mechanistically, we found that Akt1 modulates pulmonary remodelling via regulation of thrombospondin1 (TSP1) expression. Hypoxic Akt1(-) (/) (-) mice lungs expressed less TSP1. Moreover, TSP1(-) (/) (-) mice were resistant to adMyrAkt1-induced pulmonary fibrosis.
Conclusions and implications: Our study identified Akt1 as a novel target for the treatment of interstitial lung disease and provides preclinical data on the potential benefits of the Akt inhibitor triciribine for the treatment of interstitial lung disease.
© 2015 The British Pharmacological Society.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

