Gallium Compounds Exhibit Potential as New Therapeutic Agents against Mycobacterium abscessus
- PMID: 26033732
- PMCID: PMC4505262
- DOI: 10.1128/AAC.00331-15
Gallium Compounds Exhibit Potential as New Therapeutic Agents against Mycobacterium abscessus
Abstract
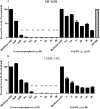
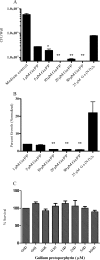
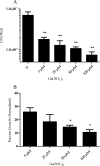
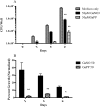
The rapidly growing nontuberculous mycobacterial species Mycobacterium abscessus has recently emerged as an important pathogen in patients with cystic fibrosis (CF). Treatment options are limited because of the organism's innate resistance to standard antituberculous antibiotics, as well as other currently available antibiotics. New antibiotic approaches to the treatment of M. abscessus are urgently needed. The goal of the present study was to assess the growth-inhibitory activity of different Ga compounds against an American Type Culture Collection (ATCC) strain and clinical isolates of M. abscessus obtained from CF and other patients. In our results, using Ga(NO3)3 and all of the other Ga compounds tested inhibited the growth of ATCC 19977 and clinical isolates of M. abscessus. Inhibition was mediated by disrupting iron uptake, as the addition of exogenous iron (Fe) restored basal growth. There were modest differences in inhibition among the isolates for the same Ga chelates, and for most Ga chelates there was only a slight difference in potency from Ga(NO3)3. In contrast, Ga-protoporphyrin completely and significantly inhibited the ATCC strain and clinical isolates of M. abscessus at much lower concentrations than Ga(NO3)3. In in vitro broth culture, Ga-protoporphyrin was more potent than Ga(NO3)3. When M. abscessus growth inside the human macrophage THP-1 cell line was assessed, Ga-protoporphyrin was >20 times more active than Ga(NO3)3. The present work suggests that Ga exhibits potent growth-inhibitory capacity against the ATCC strain, as well as against antibiotic-resistant clinical isolates of M. abscessus, including the highly antibiotic-resistant strain MC2638. Ga-based therapy offers the potential for further development as a novel therapy against M. abscessus.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

