TXA709, an FtsZ-Targeting Benzamide Prodrug with Improved Pharmacokinetics and Enhanced In Vivo Efficacy against Methicillin-Resistant Staphylococcus aureus
- PMID: 26033735
- PMCID: PMC4505295
- DOI: 10.1128/AAC.00708-15
TXA709, an FtsZ-Targeting Benzamide Prodrug with Improved Pharmacokinetics and Enhanced In Vivo Efficacy against Methicillin-Resistant Staphylococcus aureus
Abstract
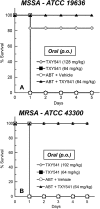
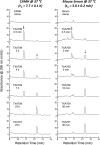
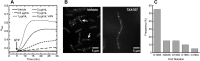
The clinical development of FtsZ-targeting benzamide compounds like PC190723 has been limited by poor drug-like and pharmacokinetic properties. Development of prodrugs of PC190723 (e.g., TXY541) resulted in enhanced pharmaceutical properties, which, in turn, led to improved intravenous efficacy as well as the first demonstration of oral efficacy in vivo against both methicillin-sensitive Staphylococcus aureus (MSSA) and methicillin-resistant S. aureus (MRSA). Despite being efficacious in vivo, TXY541 still suffered from suboptimal pharmacokinetics and the requirement of high efficacious doses. We describe here the design of a new prodrug (TXA709) in which the Cl group on the pyridyl ring has been replaced with a CF3 functionality that is resistant to metabolic attack. As a result of this enhanced metabolic stability, the product of the TXA709 prodrug (TXA707) is associated with improved pharmacokinetic properties (a 6.5-fold-longer half-life and a 3-fold-greater oral bioavailability) and superior in vivo antistaphylococcal efficacy relative to PC190723. We validate FtsZ as the antibacterial target of TXA707 and demonstrate that the compound retains potent bactericidal activity against S. aureus strains resistant to the current standard-of-care drugs vancomycin, daptomycin, and linezolid. These collective properties, coupled with minimal observed toxicity to mammalian cells, establish the prodrug TXA709 as an antistaphylococcal agent worthy of clinical development.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
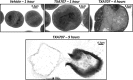

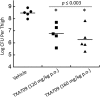
References
-
- Centers for Disease Control and Prevention. September 2013. Antibiotic resistance threats in the United States, 2013. CDC, Atlanta, GA: http://www.cdc.gov/drugresistance/threat-report-2013/.
-
- Hanaki H, Cui L, Ikeda-Dantsuji Y, Nakae T, Honda J, Yanagihara K, Takesue Y, Matsumoto T, Sunakawa K, Kaku M, Tomono K, Fukuchi K, Kusachi S, Mikamo H, Takata T, Otsuka Y, Nagura O, Fujitani S, Aoki Y, Yamaguchi Y, Tateda K, Kadota J, Kohno S, Niki Y. 2014. Antibiotic susceptibility survey of blood-borne MRSA isolates in Japan from 2008 through 2011. J Infect Chemother 20:527–534. doi:10.1016/j.jiac.2014.06.012. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

