Methotrexate, Doxorubicin, and Cisplatin (MAP) Plus Maintenance Pegylated Interferon Alfa-2b Versus MAP Alone in Patients With Resectable High-Grade Osteosarcoma and Good Histologic Response to Preoperative MAP: First Results of the EURAMOS-1 Good Response Randomized Controlled Trial
- PMID: 26033801
- PMCID: PMC4486345
- DOI: 10.1200/JCO.2014.60.0734
Methotrexate, Doxorubicin, and Cisplatin (MAP) Plus Maintenance Pegylated Interferon Alfa-2b Versus MAP Alone in Patients With Resectable High-Grade Osteosarcoma and Good Histologic Response to Preoperative MAP: First Results of the EURAMOS-1 Good Response Randomized Controlled Trial
Erratum in
-
ERRATA.J Clin Oncol. 2016 Nov 20;34(33):4059. doi: 10.1200/JCO.2016.70.9923. J Clin Oncol. 2016. PMID: 29236598 Free PMC article. No abstract available.
Abstract
Purpose: EURAMOS-1, an international randomized controlled trial, investigated maintenance therapy with pegylated interferon alfa-2b (IFN-α-2b) in patients whose osteosarcoma showed good histologic response (good response) to induction chemotherapy.
Patients and methods: At diagnosis, patients age ≤ 40 years with resectable high-grade osteosarcoma were registered. Eligibility after surgery for good response random assignment included ≥ two cycles of preoperative MAP (methotrexate, doxorubicin, and cisplatin), macroscopically complete surgery of primary tumor, < 10% viable tumor, and no disease progression. These patients were randomly assigned to four additional cycles MAP with or without IFN-α-2b (0.5 to 1.0 μg/kg per week subcutaneously, after chemotherapy until 2 years postregistration). Outcome measures were event-free survival (EFS; primary) and overall survival and toxicity (secondary).
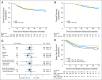
Results: Good response was reported in 1,041 of 2,260 registered patients; 716 consented to random assignment (MAP, n = 359; MAP plus IFN-α-2b, n = 357), with baseline characteristics balanced by arm. A total of 271 of 357 started IFN-α-2b; 105 stopped early, and 38 continued to receive treatment at data freeze. Refusal and toxicity were the main reasons for never starting IFN-α-2b and for stopping prematurely, respectively. Median IFN-α-2b duration, if started, was 67 weeks. A total of 133 of 268 patients who started IFN-α-2b and provided toxicity information reported grade ≥ 3 toxicity during IFN-α-2b treatment. With median follow-up of 44 months, 3-year EFS for all 716 randomly assigned patients was 76% (95% CI, 72% to 79%); 174 EFS events were reported (MAP, n = 93; MAP plus IFN-α-2b, n = 81). Hazard ratio was 0.83 (95% CI, 0.61 to 1.12; P = .214) from an adjusted Cox model.
Conclusion: At the preplanned analysis time, MAP plus IFN-α-2b was not statistically different from MAP alone. A considerable proportion of patients never started IFN-α-2b or stopped prematurely. Long-term follow-up for events and survival continues.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures

References
-
- Gorlick R, Bielack S, Teot L, et al. Osteosarcoma: Biology, diagnosis, treatment, and remaining challenges. In: Pizzo P, Poplack D, editors. Principles and Practice of Pediatric Oncology (ed 6) Philadelphia, PA: Lippincott Williams and Wilkins; 2011. pp. 1015–1044.
-
- Stiller CA, Bielack SS, Jundt G, et al. Bone tumours in European children and adolescents, 1978-1997: Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2124–2135. - PubMed
-
- Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. - PubMed
-
- Bielack S, Jürgens H, Jundt G, et al. Osteosarcoma: The COSS experience. Cancer Treat Res. 2009;152:289–308. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

