The Development of a Continuous Intravascular Glucose Monitoring Sensor
- PMID: 26033921
- PMCID: PMC4525654
- DOI: 10.1177/1932296815587937
The Development of a Continuous Intravascular Glucose Monitoring Sensor
Abstract
Background: Glycemic control in hospital intensive care units (ICU) has been the subject of numerous research publications and debate over the past 2 decades. There have been multiple studies showing the benefit of ICU glucose control in reducing both morbidity and mortality. GlySure Ltd has developed a glucose monitor based on a diboronic acid receptor that can continuously measure plasma glucose concentrations directly in a patient's vascular system. The goal of this study was to validate the performance of the GlySure CIGM system in different patient populations.
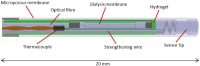
Methods: The GlySure Continuous Intravascular Glucose Monitoring (CIGM) System was evaluated in both the Cardiac ICU (33 patients) and MICU setting (14 patients). The sensor was placed through a custom CVC and measured the patients' blood glucose concentration every 15 seconds. Comparison blood samples were taken at 2 hourly then 4 hourly intervals and measured on a YSI 2300 STAT Plus or an i-STAT.
Results: Consensus error grid analysis of the data shows that the majority of the data (88.2% Cardiac, and 95.0% MICU) fell within zone A, which is considered to be clinically accurate and all data points fell within zones A and B. The MARD of the Cardiac trial was 9.90% and the MICU trial had a MARD of 7.95%. Data analysis showed no significant differences between data generated from Cardiac and MICU patients or by time or glucose concentration.
Conclusions: The GlySure CIGM System has met the design challenges of measuring intravascular glucose concentrations in critically ill patients with acceptable safety and performance criteria and without disrupting current clinical practice. The accuracy of the data is not affected by the patients' condition.
Keywords: boronic acid; continuous glucose; intravascular.
© 2015 Diabetes Technology Society.
Conflict of interest statement
Figures

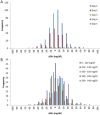
 ) based on intermittent blood sample measurements (
) based on intermittent blood sample measurements ( ); samples 7 and 8 are highlighted (
); samples 7 and 8 are highlighted ( ). (B) CGM (-) versus intermittent blood sample measurements.
). (B) CGM (-) versus intermittent blood sample measurements.

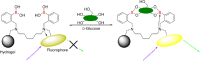




References
-
- Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-1367. - PubMed
-
- Krinsley J. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79:992-1000. - PubMed
-
- Kanji S. Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med. 2005;33:2778-2785. - PubMed
-
- Cembrowskia GS, Tran DV, Slater-Maclean L, et al. Could susceptibility to low hematocrit interference have compromised the results of the NICE-SUGAR Trial? Clin Chem. 2010;55:1193-1195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

