Loss of MyD88 alters neuroinflammatory response and attenuates early Purkinje cell loss in a spinocerebellar ataxia type 6 mouse model
- PMID: 26034136
- PMCID: PMC4527484
- DOI: 10.1093/hmg/ddv202
Loss of MyD88 alters neuroinflammatory response and attenuates early Purkinje cell loss in a spinocerebellar ataxia type 6 mouse model
Abstract
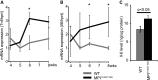
Spinocerebellar ataxia type 6 (SCA6) is dominantly inherited neurodegenerative disease, caused by an expansion of CAG repeat encoding a polyglutamine (PolyQ) tract in the Cav2.1 voltage-gated calcium channel. Its key pathological features include selective degeneration of the cerebellar Purkinje cells (PCs), a common target for PolyQ-induced toxicity in various SCAs. Mutant Cav2.1 confers toxicity primarily through a toxic gain-of-function mechanism; however, its molecular basis remains elusive. Here, we studied the cerebellar gene expression patterns of young Sca6-MPI(118Q/118Q) knockin (KI) mice, which expressed mutant Cav2.1 from an endogenous locus and recapitulated many phenotypic features of human SCA6. Transcriptional signatures in the MPI(118Q/118Q) mice were distinct from those in the Sca1(154Q/2Q) mice, a faithful SCA1 KI mouse model. Temporal expression profiles of the candidate genes revealed that the up-regulation of genes associated with microglial activation was initiated before PC degeneration and was augmented as the disease progressed. Histological analysis of the MPI(118Q/118Q) cerebellum showed the predominance of M1-like pro-inflammatory microglia and it was concomitant with elevated expression levels of tumor necrosis factor, interleukin-6, Toll-like receptor (TLR) 2 and 7. Genetic ablation of MyD88, a major adaptor protein conveying TLR signaling, altered expression patterns of M1/M2 microglial phenotypic markers in the MPI(118Q/118Q) cerebellum. More importantly, it ameliorated PC loss and partially rescued motor impairments in the early disease phase. These results suggest that early neuroinflammatory response may play an important role in the pathogenesis of SCA6 and its modulation could pave the way for slowing the disease progression during the early stage of the disease.
© The Author 2015. Published by Oxford University Press.
Figures




References
-
- Orr H.T., Zoghbi H.Y. (2007) Trinucleotide repeat disorders. Annu. Rev. Neurosci., 30, 575–621.2. - PubMed
-
- Zhuchenko O., Bailey J., Bonnen P., Ashizawa T., Stockton D.W., Amos C., Dobyns W.B., Subramony S.H., Zoghbi H.Y., Lee C.C. (1997) Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha(1A)-voltage-dependent calcium channel. Nat. Genet., 15, 62–69. - PubMed
-
- Ishikawa K., Tanaka H., Saito M., Ohkoshi N., Fujita T., Yoshizawa K., Ikeuchi T., Watanabe M., Hayashi A., Takiyama Y. et al. (1997) Japanese families with autosomal dominant pure cerebellar ataxia map to chromosome 19p13.1-p13.2 and are strongly associated with mild CAG expansions in the spinocerebellar ataxia type 6 gene in chromosome 19p13.1. Am. J. Hum. Genet., 61, 336–346. - PMC - PubMed
-
- Watase K., Barrett C.F., Miyazaki T., Ishiguro T., Ishikawa K., Hu Y., Unno T., Sun Y., Kasai S., Watanabe M. et al. (2008) Spinocerebellar ataxia type 6 knockin mice develop a progressive neuronal dysfunction with age-dependent accumulation of mutant Ca(v)2.1 channels. Proc. Natl Acad. Sci. U.S.A., 105, 11987–11992. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

