Scalable syntheses of the BET bromodomain inhibitor JQ1
- PMID: 26034331
- PMCID: PMC4448696
- DOI: 10.1016/j.tetlet.2015.02.062
Scalable syntheses of the BET bromodomain inhibitor JQ1
Abstract
We have developed methods involving the use of alternate, safer reagents for the scalable syntheses of the potent BET bromodomain inhibitor JQ1. A one-pot three step method, involving the conversion of a benzodiazepine to a thioamde using Lawesson's reagent, followed by amidrazone formation and installation of the triazole moiety furnished JQ1. This method provides good yields and a facile purification process. For the synthesis of enantiomerically enriched (+)-JQ1, the highly toxic reagent diethyl chlorophosphate, used in a previous synthesis, was replaced with the safer reagent diphenyl chlorophosphate in the three-step one-pot triazole formation without effecting yields and enantiomeric purity of (+)-JQ1.
Keywords: BET inhibitors; Bromodomains; Male contraceptive; One-pot method; Thionation; Triazolothienodiazepine (+)-JQ1.
Figures
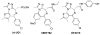
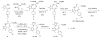
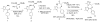



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
