Non-invasive sources of cells with primary cilia from pediatric and adult patients
- PMID: 26034581
- PMCID: PMC4450497
- DOI: 10.1186/s13630-015-0017-x
Non-invasive sources of cells with primary cilia from pediatric and adult patients
Abstract
Background: Ciliopathies give rise to a multitude of organ-specific pathologies; obtaining relevant primary patient material is useful for both diagnostics and research. However, acquisition of primary ciliated cells from patients, particularly pediatric patients, presents multiple difficulties. Biopsies and blood samples are invasive, and patients (and their parents) may be reluctant to travel to medical centers, especially for research purposes. We sought to develop non-invasive methods of obtaining viable and ciliated primary cells from ciliopathy patients which could be obtained in the home environment.
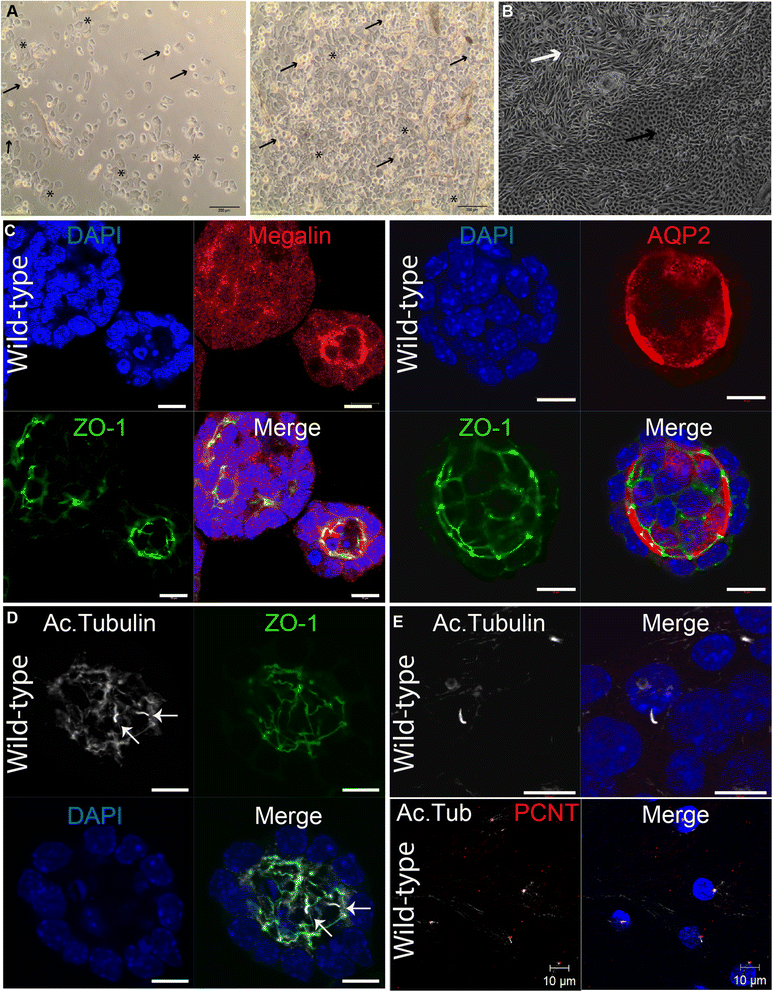
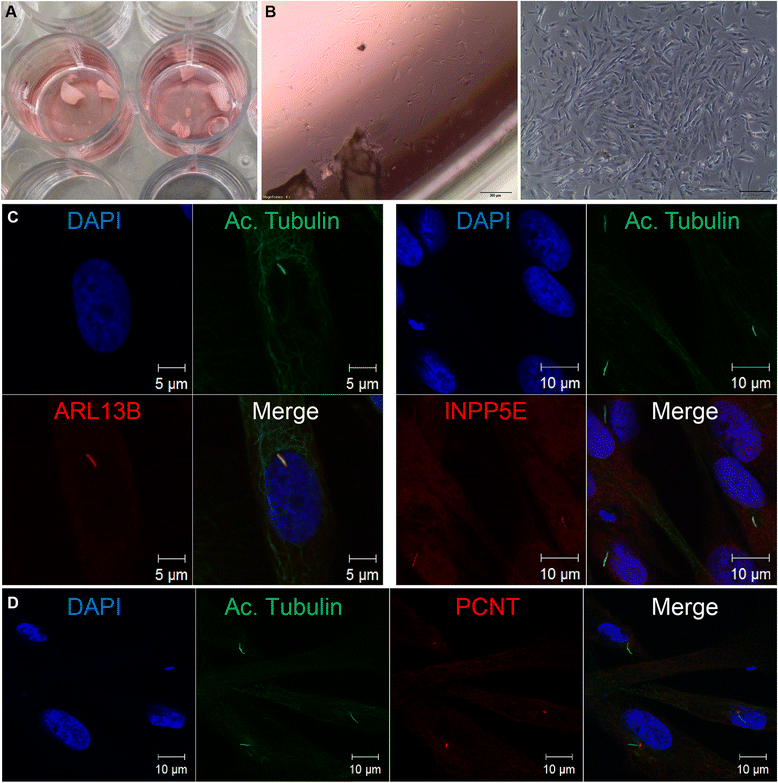
Findings: We introduce two methods for the non-invasive acquisition of primary ciliated cells. In one approach, we collected spontaneously shed deciduous (milk) teeth from children. Fibroblast-like cells were observed after approximately 2 weeks of culture of fragmented teeth. Secondly, urine samples were collected from children or adults. Cellular content was isolated and after approximately 1 week, renal epithelial cells were observed. Both urine and tooth-derived cells ciliate and express ciliary proteins visible with immunofluorescence. Urine-derived renal epithelial cells (URECs) are amenable to 3D culturing, siRNA knockdown, and ex vivo drug testing.
Conclusions: As evidence continues to accumulate showing that the primary cilium has a central role in development and disease, the need for readily available and ciliated patient cells will increase. Here, we introduce two methods for the non-invasive acquisition of cells with primary cilia. We believe that these cells can be used for further ex vivo study of ciliopathies and in the future, for personalized medicine.
Keywords: Cell culture; Cilia; Ciliopathy; Deciduous tooth; Pediatrics; Protocol; Urine.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

