Comparative effectiveness of induction therapy for human immunodeficiency virus-associated cryptococcal meningitis: a network meta-analysis
- PMID: 26034761
- PMCID: PMC4438891
- DOI: 10.1093/ofid/ofv010
Comparative effectiveness of induction therapy for human immunodeficiency virus-associated cryptococcal meningitis: a network meta-analysis
Abstract
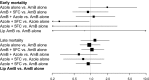
Background. Multiple international treatment guidelines recommend amphotericin-based combination regimens for induction therapy of cryptococcal meningitis. Yet, only 1 trial has reported a mortality benefit for combination amphotericin-flucytosine, and none have reported a mortality benefit for combination amphotericin-fluconazole. Methods. We conducted a Bayesian network meta-analysis to estimate the comparative effectiveness of recommended induction therapies for HIV-associated cryptococcal meningitis. We searched PubMed and Cochrane CENTRAL for clinical reports of induction therapy for HIV-associated cryptococcal meningitis. We extracted or calculated early (two-week) and late (six to 12-week) mortality by treatment arm for the following induction regimens: amphotericin B alone, amphotericin B + flucytosine, amphotericin B + triazoles, amphotericin B + flucytosine +triazoles, triazoles alone, triazoles + flucytosine, liposomal amphotericin B, and amphotericin B + other medicines. Results. In the overall sample (35 studies, n = 2483), we found no evidence of decreased mortality from addition of flucytosine or triazoles to amphotericin B, compared with amphotericin B alone. Although we did find a nonsignificant benefit for addition of flucytosine to amphotericin B in studies including participants with altered levels of consciousness, we did not identify a benefit for combination therapy in restricted analyses in either resource-rich or resource-limited settings, studies conducted before or after 2004, and studies restricted to a high dose of amphotericin B and fluconazole. Conclusions. Given considerations of drug availability and toxicity, there is an important need for additional data to clarify which populations are most likely to benefit from combination therapies for human immunodeficiency virus-associated cryptococcal meningitis.
Keywords: HIV/AIDS; cryptococcal meningitis; induction therapy; network meta-analysis; therapeutics.
Figures




References
-
- Liechty CA, Solberg P, Were W, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health. 2007;12:929–35. - PubMed
-
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–30. - PubMed
-
- French N, Gray K, Watera C, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002;16:1031–8. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

