Deducing the stage of origin of Wilms' tumours from a developmental series of Wt1-mutant mice
- PMID: 26035382
- PMCID: PMC4527280
- DOI: 10.1242/dmm.018523
Deducing the stage of origin of Wilms' tumours from a developmental series of Wt1-mutant mice
Abstract
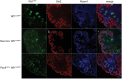
Wilms' tumours, paediatric kidney cancers, are the archetypal example of tumours caused through the disruption of normal development. The genetically best-defined subgroup of Wilms' tumours is the group caused by biallelic loss of the WT1 tumour suppressor gene. Here, we describe a developmental series of mouse models with conditional loss of Wt1 in different stages of nephron development before and after the mesenchymal-to-epithelial transition (MET). We demonstrate that Wt1 is essential for normal development at all kidney developmental stages under study. Comparison of genome-wide expression data from the mutant mouse models with human tumour material of mutant or wild-type WT1 datasets identified the stage of origin of human WT1-mutant tumours, and emphasizes fundamental differences between the two human tumour groups due to different developmental stages of origin.
Keywords: Kidney development; Mouse model; WT1; Wilms’ tumour.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures






References
-
- Bancroft J. D. and Stevens A. (1990). Theory and Practice of Histological Techniques. Edinburgh: Churchill Linvingstone.
-
- Brunskill E. W., Aronow B. J., Georgas K., Rumballe B., Valerius M. T., Aronow J., Kaimal V., Jegga A. G., Grimmond S., McMahon A. P. et al. (2008). Atlas of gene expression in the developing kidney at microanatomic resolution. Dev. Cell 15, 781-791. 10.1016/j.devcel.2008.09.007 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

