Phospholamban overexpression in mice causes a centronuclear myopathy-like phenotype
- PMID: 26035394
- PMCID: PMC4527296
- DOI: 10.1242/dmm.020859
Phospholamban overexpression in mice causes a centronuclear myopathy-like phenotype
Abstract
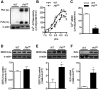
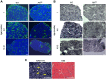
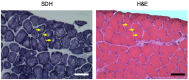
Centronuclear myopathy (CNM) is a congenital myopathy that is histopathologically characterized by centrally located nuclei, central aggregation of oxidative activity, and type I fiber predominance and hypotrophy. Here, we obtained commercially available mice overexpressing phospholamban (Pln(OE)), a well-known inhibitor of sarco(endo)plasmic reticulum Ca(2+)-ATPases (SERCAs), in their slow-twitch type I skeletal muscle fibers to determine the effects on SERCA function. As expected with a 6- to 7-fold overexpression of phospholamban, SERCA dysfunction was evident in Pln(OE) muscles, with marked reductions in rates of Ca(2+) uptake, maximal ATPase activity and the apparent affinity of SERCA for Ca(2+). However, our most significant discovery was that the soleus and gluteus minimus muscles from the Pln(OE) mice displayed overt signs of myopathy: they histopathologically resembled human CNM, with centrally located nuclei, central aggregation of oxidative activity, type I fiber predominance and hypotrophy, progressive fibrosis and muscle weakness. This phenotype is associated with significant upregulation of muscle sarcolipin and dynamin 2, increased Ca(2+)-activated proteolysis, oxidative stress and protein nitrosylation. Moreover, in our assessment of muscle biopsies from three human CNM patients, we found a significant 53% reduction in SERCA activity and increases in both total and monomeric PLN content compared with five healthy subjects, thereby justifying future studies with more CNM patients. Altogether, our results suggest that the commercially available Pln(OE) mouse phenotypically resembles human CNM and could be used as a model to test potential mechanisms and therapeutic strategies. To date, there is no cure for CNM and our results suggest that targeting SERCA function, which has already been shown to be an effective therapeutic target for murine muscular dystrophy and human cardiomyopathy, might represent a novel therapeutic strategy to combat CNM.
Keywords: Calcium regulation; Congenital myopathy; Dynamin 2; SERCA; Skeletal muscle.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures


References
-
- Agrawal P. B., Pierson C. R., Joshi M., Liu X., Ravenscroft G., Moghadaszadeh B., Talabere T., Viola M., Swanson L. C., Haliloğlu G. et al. (2014). SPEG interacts with myotubularin, and its deficiency causes centronuclear myopathy with dilated cardiomyopathy. Am. J. Hum. Genet. 95, 218-226. 10.1016/j.ajhg.2014.07.004 - DOI - PMC - PubMed
-
- Al-Qusairi L., Weiss N., Toussaint A., Berbey C., Messaddeq N., Kretz C., Sanoudou D., Beggs A. H., Allard B., Mandel J.-L. et al. (2009). T-tubule disorganization and defective excitation-contraction coupling in muscle fibers lacking myotubularin lipid phosphatase. Proc. Natl. Acad. Sci. USA 106, 18763-18768. 10.1073/pnas.0900705106 - DOI - PMC - PubMed
-
- Altamirano F., Lopez J. R., Henriquez C., Molinski T., Allen P. D. and Jaimovich E. (2012). Increased resting intracellular calcium modulates NF-kappaB-dependent inducible nitric-oxide synthase gene expression in dystrophic mdx skeletal myotubes. J. Biol. Chem. 287, 20876-20887. 10.1074/jbc.M112.344929 - DOI - PMC - PubMed
-
- Anderson D. M., Anderson K. M., Chang C.-L., Makarewich C. A., Nelson B. R., McAnally J. R., Kasaragod P., Shelton J. M., Liou J., Bassel-Duby R. et al. (2015). A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell 160, 595-606. 10.1016/j.cell.2015.01.009 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

