Heterogeneous disease progression and treatment response in a C3HeB/FeJ mouse model of tuberculosis
- PMID: 26035868
- PMCID: PMC4457036
- DOI: 10.1242/dmm.019513
Heterogeneous disease progression and treatment response in a C3HeB/FeJ mouse model of tuberculosis
Abstract
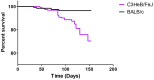
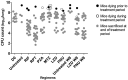
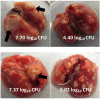
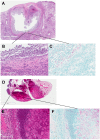
Mice are the most commonly used species for non-clinical evaluations of drug efficacy against tuberculosis (TB). Unlike commonly used strains, C3HeB/FeJ mice develop caseous necrosis in the lung, which might alter the representation of drug efficacy in a way that is more like human TB. Because the development of such pathology requires time, we investigated the effect of infection incubation period on the activity of six drugs in C3HeB/FeJ and BALB/c mice. Mice were aerosol infected and held for 6, 10 or 14 weeks before receiving therapy with rifampin (RIF), rifapentine (RPT), pyrazinamide (PZA), linezolid (LZD), sutezolid (PNU) or metronidazole (MTZ) for 4-8 weeks. Outcomes included pathological assessments, pH measurements of liquefied caseum and assessment of colony-forming unit (CFU) counts from lung cultures. Remarkable heterogeneity in the timing and extent of disease progression was observed in C3HeB/FeJ mice, largely independent of incubation period. Likewise, drug efficacy in C3HeB/FeJ mice was not affected by incubation period. However, for PZA, LZD and PNU, dichotomous treatment effects correlating with the presence or absence of large caseous lesions were observed. In the case of PZA, its poor activity in the subset of C3HeB/FeJ mice with large caseous lesions might be explained by the pH of 7.36±0.09 measured in liquefied caseum. This study highlights the potential value of C3HeB/FeJ mice for non-clinical efficacy testing, especially for investigating the interaction of lesion pathology and drug effect. Careful use of this model could enhance the bridging of non-clinical results with clinical outcomes.
Keywords: C3HeB/FeJ; Heterogeneity; Pyrazinamide; Tuberculosis; pH.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
E.L.N. is co-inventor on the following patent involving sutezolid: US2011190199 “Combination Therapy for Tuberculosis”. J.-P.L. and A.J.L. have no competing interests.
Figures


References
-
- Benator D., Bhattacharya M., Bozeman L., Burman W., Cantazaro A., Chaisson R., Gordin F., Horsburgh C. R., Horton J., Khan A. et al. (2002). Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet 9332, 528-534. - PubMed
-
- Canetti G. (1955). The tubercle bacillus in the pulmonary lesion. In The Tubercle Bacillus in the Pulmonary Lesion of Man. pp. 29-85. New York: Springer Publishing Company.
-
- Chew W. K., Segarra I., Ambu S. and Mak J. W. (2012). Significant reduction of brain cysts caused by Toxoplasma gondii after treatment with spiramycin coadministered with metronidazole in a mouse model of chronic toxoplasmosis. Antimicrob. Agents Chemother. 56, 1762-1768. 10.1128/AAC.05183-11 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

