Randomized, double-blinded, placebo-controlled trial of fibrinogen concentrate supplementation after complex cardiac surgery
- PMID: 26037084
- PMCID: PMC4599543
- DOI: 10.1161/JAHA.115.002066
Randomized, double-blinded, placebo-controlled trial of fibrinogen concentrate supplementation after complex cardiac surgery
Abstract
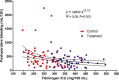
Background: Postoperative bleeding after heart operations is still a common finding, leading to allogeneic blood products transfusion. Fibrinogen and coagulation factors deficiency are possible determinants of bleeding. The experimental hypothesis of this study is that a first-line fibrinogen supplementation avoids the need for fresh frozen plasma (FFP) and reduces the need for any kind of transfusions.
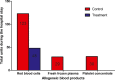
Methods and results: This was a single-center, prospective, randomized, placebo-controlled, double-blinded study. One-hundred sixteen patients undergoing heart surgery with an expected cardiopulmonary bypass duration >90 minutes were admitted to the study. Patients in the treatment arm received fibrinogen concentrate after protamine administration; patients in the control arm received saline solution. In case of ongoing bleeding, patients in the treatment arm could receive prothrombin complex concentrates (PCCs) and those in the control arm saline solution. The primary endpoint was avoidance of any allogeneic blood product. Patients in the treatment arm had a significantly lower rate of any allogeneic blood products transfusion (odds ratio, 0.40; 95% confidence interval, 0.19 to 0.84, P=0.015). The total amount of packed red cells and FFP units transfused was significantly lower in the treatment arm. Postoperative bleeding was significantly (P=0.042) less in the treatment arm (median, 300 mL; interquartile range, 200 to 400 mL) than in the control arm (median, 355 mL; interquartile range, 250 to 600 mL).
Conclusions: Fibrinogen concentrate limits postoperative bleeding after complex heart surgery, leading to a significant reduction in allogeneic blood products transfusions. No safety issues were raised.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT01471730.
Keywords: cardiopulmonary bypass; fibrinogen; hemorrhage; surgery.
© 2015 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures
References
-
- Puetz J. Fresh frozen plasma: the most commonly prescribed hemostatic agent. J Thromb Haemost. 2013;11:1794–1799. - PubMed
-
- Stanworth SJ, Grant-Casey J, Lowe D, Laffan M, New H, Murphy MF, Allard S. The use of fresh-frozen plasma in England: high levels of inappropriate use in adults and children. Transfusion. 2011;51:62–70. - PubMed
-
- US Department of Health and Human Services. The 2009 National Blood Collection and Utilization Survey Report. Washington, DC: US Department of Health and Human Services, Office of the Assistant Secretary for Health; 2011. . Available at: http://www.hhs.gov/ash/bloodsafety/2011-nbcus.pdf. Accessed December 16, 2014.
-
- Vonk AB, Meesters MI, van Dijk WB, Eijsman L, Romijn JW, Jansen EK, Loer SA, Boer C. Ten-year patterns in blood product utilization during cardiothoracic surgery with cardiopulmonary bypass in a tertiary hospital. Transfusion. 2014;54:2608–2616. - PubMed
-
- Ternstrom L, Radulovic V, Karlsson M, Baghaei F, Hyllner M, Bylock A, Hansson KM, Jeppsson A. Plasma activity of individual coagulation factors, hemodilution and blood loss after cardiac surgery: a prospective observational study. Thromb Res. 2010;126:e128–e133. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

