Genomic Context of Azole Resistance Mutations in Aspergillus fumigatus Determined Using Whole-Genome Sequencing
- PMID: 26037120
- PMCID: PMC4453006
- DOI: 10.1128/mBio.00536-15
Genomic Context of Azole Resistance Mutations in Aspergillus fumigatus Determined Using Whole-Genome Sequencing
Erratum in
-
Erratum for Abdolrasouli et al., Genomic Context of Azole Resistance Mutations in Aspergillus fumigatus Determined Using Whole-Genome Sequencing.mBio. 2015 Jul 14;6(4):e00939. doi: 10.1128/mBio.00939-15. mBio. 2015. PMID: 26173700 Free PMC article. No abstract available.
Abstract
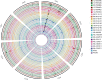
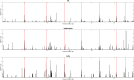
A rapid and global emergence of azole resistance has been observed in the pathogenic fungus Aspergillus fumigatus over the past decade. The dominant resistance mechanism appears to be of environmental origin and involves mutations in the cyp51A gene, which encodes a protein targeted by triazole antifungal drugs. Whole-genome sequencing (WGS) was performed for high-resolution single-nucleotide polymorphism (SNP) analysis of 24 A. fumigatus isolates, including azole-resistant and susceptible clinical and environmental strains obtained from India, the Netherlands, and the United Kingdom, in order to assess the utility of WGS for characterizing the alleles causing resistance. WGS analysis confirmed that TR34/L98H (a mutation comprising a tandem repeat [TR] of 34 bases in the promoter of the cyp51A gene and a leucine-to-histidine change at codon 98) is the sole mechanism of azole resistance among the isolates tested in this panel of isolates. We used population genomic analysis and showed that A. fumigatus was panmictic, with as much genetic diversity found within a country as is found between continents. A striking exception to this was shown in India, where isolates are highly related despite being isolated from both clinical and environmental sources across >1,000 km; this broad occurrence suggests a recent selective sweep of a highly fit genotype that is associated with the TR34/L98H allele. We found that these sequenced isolates are all recombining, showing that azole-resistant alleles are segregating into diverse genetic backgrounds. Our analysis delineates the fundamental population genetic parameters that are needed to enable the use of genome-wide association studies to identify the contribution of SNP diversity to the generation and spread of azole resistance in this medically important fungus.
Importance: Resistance to azoles in the ubiquitous ascomycete fungus A. fumigatus was first reported from clinical isolates collected in the United States during the late 1980s. Over the last decade, an increasing number of A. fumigatus isolates from the clinic and from nature have been found to show resistance to azoles, suggesting that resistance is emerging through selection by the widespread usage of agricultural azole antifungal compounds. Aspergillosis is an emerging clinical problem, with high rates of treatment failures necessitating the development of new techniques for surveillance and for determining the genome-wide basis of azole resistance in A. fumigatus.
Copyright © 2015 Abdolrasouli et al.
Figures

References
-
- Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik J-A, Wingard JR, Patterson TF, Infectious Diseases Society of America . 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 46:327–360. doi:10.1086/525258. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
