Defining the Roles of TcdA and TcdB in Localized Gastrointestinal Disease, Systemic Organ Damage, and the Host Response during Clostridium difficile Infections
- PMID: 26037121
- PMCID: PMC4453007
- DOI: 10.1128/mBio.00551-15
Defining the Roles of TcdA and TcdB in Localized Gastrointestinal Disease, Systemic Organ Damage, and the Host Response during Clostridium difficile Infections
Abstract
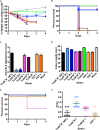
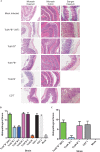
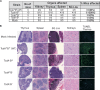
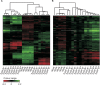
Clostridium difficile is a leading cause of antibiotic-associated diarrhea, a significant animal pathogen, and a worldwide public health burden. Most disease-causing strains secrete two exotoxins, TcdA and TcdB, which are considered to be the primary virulence factors. Understanding the role that these toxins play in disease is essential for the rational design of urgently needed new therapeutics. However, their relative contributions to disease remain contentious. Using three different animal models, we show that TcdA(+) TcdB(-) mutants are attenuated in virulence in comparison to the wild-type (TcdA(+) TcdB(+)) strain, whereas TcdA(-) TcdB(+) mutants are fully virulent. We also show for the first time that TcdB alone is associated with both severe localized intestinal damage and systemic organ damage, suggesting that this toxin might be responsible for the onset of multiple organ dysfunction syndrome (MODS), a poorly characterized but often fatal complication of C. difficile infection (CDI). Finally, we show that TcdB is the primary factor responsible for inducing the in vivo host innate immune and inflammatory responses. Surprisingly, the animal infection model used was found to profoundly influence disease outcomes, a finding which has important ramifications for the validation of new therapeutics and future disease pathogenesis studies. Overall, our results show unequivocally that TcdB is the major virulence factor of C. difficile and provide new insights into the host response to C. difficile during infection. The results also highlight the critical nature of using appropriate and, when possible, multiple animal infection models when studying bacterial virulence mechanisms.
Importance: Clostridium difficile is a leading cause of antibiotic-associated diarrhea and an important hospital pathogen. TcdA and TcdB are thought to be the primary virulence factors responsible for disease symptoms of C. difficile infections (CDI). However, the individual contributions of these toxins to disease remain contentious. Using three different animal models of infection, we show for the first time that TcdB alone causes severe damage to the gut, as well as systemic organ damage, suggesting that this toxin might be responsible for MODS, a serious but poorly understood complication of CDI. These findings provide important new insights into the host response to C. difficile during infection and should guide the rational development of urgently required nonantibiotic therapeutics for the treatment of CDI.
Copyright © 2015 Carter et al.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
