Astrocytes regulate α-secretase-cleaved soluble amyloid precursor protein secretion in neuronal cells: Involvement of group IIA secretory phospholipase A2
- PMID: 26037803
- PMCID: PMC4485573
- DOI: 10.1016/j.neuroscience.2015.05.052
Astrocytes regulate α-secretase-cleaved soluble amyloid precursor protein secretion in neuronal cells: Involvement of group IIA secretory phospholipase A2
Abstract
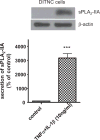
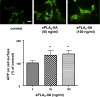
Astrocytes are major supportive cells in brains with important functions including providing nutrients and regulating neuronal activities. In this study, we demonstrated that astrocytes regulate amyloid precursor protein (APP) processing in neuronal cells through secretion of group IIA secretory phospholipase A2 (sPLA2-IIA). When astrocytic cells (DITNC) were mildly stimulated with the pro-inflammatory cytokines, such as TNF α and IL-1β, sPLA2-IIA was secreted into the medium. When conditioned medium containing sPLA2-IIA was applied to human neuroblastoma (SH-SY5Y) cells, there was an increase in both cell membrane fluidity and secretion of α-secretase-cleaved soluble amyloid precursor protein (sAPPα). These changes were abrogated by KH064, a selective inhibitor of sPLA2-IIA. In addition, exposing SH-SY5Y cells to recombinant human sPLA2-IIA also increased membrane fluidity, accumulation of APP at the cell surface, and secretion of sAPPα, but without altering total expressions of APP, α-secretases and β-site APP cleaving enzyme (BACE1). Taken together, our results provide novel information regarding a functional role of sPLA2-IIA in astrocytes for regulating APP processing in neuronal cells.
Keywords: SH-SY5Y; astrocytes; cytokine; membrane fluidity; sAPP(α); sPLA(2)-IIA.
Copyright © 2015 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures




References
-
- Allinson TM, Parkin ET, Turner AJ, Hooper NM. ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res. 2003;74:342–352. - PubMed
-
- Apelt J, Schliebs R. Beta-amyloid-induced glial expression of both pro- and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res. 2001;894:21–30. - PubMed
-
- Baranowska-Bik A, Bik W, Wolinska-Witort E, Martynska L, Chmielowska M, Barcikowska M, Baranowska B. Plasma beta amyloid and cytokine profile in women with Alzheimer’s disease. Neuro Endocrinol Lett. 2008;29:75–79. - PubMed
-
- Beloosesky Y, Salman H, Bergman M, Bessler H, Djaldetti M. Cytokine levels and phagocytic activity in patients with Alzheimer’s disease. Gerontology. 2002;48:128–132. - PubMed
-
- Cacquevel M, Lebeurrier N, Cheenne S, Vivien D. Cytokines in neuroinflammation and Alzheimer’s disease. Curr Drug Targets. 2004;5:529–534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

