Learning From Animal Models of Obsessive-Compulsive Disorder
- PMID: 26037910
- PMCID: PMC4633402
- DOI: 10.1016/j.biopsych.2015.04.020
Learning From Animal Models of Obsessive-Compulsive Disorder
Abstract
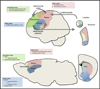
Obsessive-compulsive disorder (OCD) affects 2%-3% of the population worldwide and can cause significant distress and disability. Substantial challenges remain in the field of OCD research and therapeutics. Approved interventions alleviate symptoms only partially, with 30%-40% of patients being resistant to treatment. Although the etiology of OCD is still unknown, research evidence points toward the involvement of cortico-striato-thalamocortical circuitry. This review focuses on the most recent behavioral, genetics, and neurophysiologic findings from animal models of OCD. Based on evidence from these models and parallels with human studies, we discuss the circuit hyperactivity hypothesis for OCD, a potential circuitry dysfunction of action termination, and the involvement of candidate genes. Adding a more biologically valid framework to OCD will help researchers define and test new hypotheses and facilitate the development of targeted therapies based on disease-specific mechanisms.
Keywords: Animal models; Basal ganglia; CSTC; OCD; Obsessive-compulsive disorder; Striatum; Synapse.
Copyright © 2016 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Patricia Monteiro declares no biomedical financial interests or potential conflicts of interest.
Figures

Comment in
-
Toward Better Animal Models for Molecular Psychiatry.Biol Psychiatry. 2016 Jan 1;79(1):2-3. doi: 10.1016/j.biopsych.2015.10.019. Biol Psychiatry. 2016. PMID: 26616431 No abstract available.
-
There Is Much to Be Learned From Animal Models of Obsessive-Compulsive Disorder.Biol Psychiatry. 2016 Jan 1;79(1):e1-3. doi: 10.1016/j.biopsych.2015.10.010. Biol Psychiatry. 2016. PMID: 26616433 No abstract available.
References
-
- Fontenelle LF, Mendlowicz MV, Versiani M. The descriptive epidemiology of obsessive-compulsive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:327–337. - PubMed
-
- Fineberg Na, Gale TM. Evidence-based pharmacotherapy of obsessive-compulsive disorder. Int. J. Neuropsychopharmacol. 2005 Mar.8(1):107–129. - PubMed
-
- Pallanti S, Hollander E. Pharmacological, experimental therapeutic, and transcranial magnetic stimulation treatments for compulsivity and impulsivity. CNS Spectr. 2014 Feb.19(1):50–61. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

