A Multifaceted Study of Scedosporium boydii Cell Wall Changes during Germination and Identification of GPI-Anchored Proteins
- PMID: 26038837
- PMCID: PMC4454578
- DOI: 10.1371/journal.pone.0128680
A Multifaceted Study of Scedosporium boydii Cell Wall Changes during Germination and Identification of GPI-Anchored Proteins
Abstract
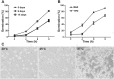
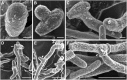
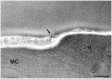
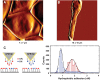
Scedosporium boydii is a pathogenic filamentous fungus that causes a wide range of human infections, notably respiratory infections in patients with cystic fibrosis. The development of new therapeutic strategies targeting S. boydii necessitates a better understanding of the physiology of this fungus and the identification of new molecular targets. In this work, we studied the conidium-to-germ tube transition using a variety of techniques including scanning and transmission electron microscopy, atomic force microscopy, two-phase partitioning, microelectrophoresis and cationized ferritin labeling, chemical force spectroscopy, lectin labeling, and nanoLC-MS/MS for cell wall GPI-anchored protein analysis. We demonstrated that the cell wall undergoes structural changes with germination accompanied with a lower hydrophobicity, electrostatic charge and binding capacity to cationized ferritin. Changes during germination also included a higher accessibility of some cell wall polysaccharides to lectins and less CH3/CH3 interactions (hydrophobic adhesion forces mainly due to glycoproteins). We also extracted and identified 20 GPI-anchored proteins from the cell wall of S. boydii, among which one was detected only in the conidial wall extract and 12 only in the mycelial wall extract. The identified sequences belonged to protein families involved in virulence in other fungi like Gelp/Gasp, Crhp, Bglp/Bgtp families and a superoxide dismutase. These results highlighted the cell wall remodeling during germination in S. boydii with the identification of a substantial number of cell wall GPI-anchored conidial or hyphal specific proteins, which provides a basis to investigate the role of these molecules in the host-pathogen interaction and fungal virulence.
Conflict of interest statement
Figures



References
-
- Guarro J, Kantarcioglu AS, Horré R, Luis Rodriguez-Tudela J, Cuenca Estrella M, Berenguer J, et al. 2006. Scedosporium apiospermum: changing clinical spectrum of a therapy-refractory opportunist. Med Mycol 44:295–327. - PubMed
-
- Harun A, Gilgado F, Chen SC, Meyer W. 2010. Abundance of Pseudallescheria/Scedosporium species in the Australian urban environment suggests a possible source for scedosporiosis including the colonization of airways in cystic fibrosis. Med Mycol 48 (Suppl 1):S70–S76. 10.3109/13693786.2010.515254 - DOI - PubMed
-
- Beguin H, Nolard N. 1994. Mould biodiversity in homes I. Air and surface analysis of 130 dwellings. Aerobiologia 10: 157–166.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

