Maternal Dexamethasone Treatment Alters Tissue and Circulating Components of the Renin-Angiotensin System in the Pregnant Ewe and Fetus
- PMID: 26039155
- PMCID: PMC4511127
- DOI: 10.1210/en.2015-1197
Maternal Dexamethasone Treatment Alters Tissue and Circulating Components of the Renin-Angiotensin System in the Pregnant Ewe and Fetus
Abstract
Antenatal synthetic glucocorticoids promote fetal maturation in pregnant women at risk of preterm delivery and their mechanism of action may involve other endocrine systems. This study investigated the effect of maternal dexamethasone treatment, at clinically relevant doses, on components of the renin-angiotensin system (RAS) in the pregnant ewe and fetus. From 125 days of gestation (term, 145 ± 2 d), 10 ewes carrying single fetuses of mixed sex (3 female, 7 male) were injected twice im, at 10-11 pm, with dexamethasone (2 × 12 mg, n = 5) or saline (n = 5) at 24-hour intervals. At 10 hours after the second injection, maternal dexamethasone treatment increased angiotensin-converting enzyme (ACE) mRNA levels in the fetal lungs, kidneys, and heart and ACE concentration in the circulation and lungs, but not kidneys, of the fetuses. Fetal cardiac mRNA abundance of angiotensin II (AII) type 2 receptor decreased after maternal dexamethasone treatment. Between the two groups of fetuses, there were no significant differences in plasma angiotensinogen or renin concentrations; in transcript levels of renal renin, or AII type 1 or 2 receptors in the lungs and kidneys; or in pulmonary, renal or cardiac protein content of the AII receptors. In the pregnant ewes, dexamethasone administration increased pulmonary ACE and plasma angiotensinogen, and decreased plasma renin, concentrations. Some of the effects of dexamethasone treatment on the maternal and fetal RAS were associated with altered insulin and thyroid hormone activity. Changes in the local and circulating RAS induced by dexamethasone exposure in utero may contribute to the maturational and tissue-specific actions of antenatal glucocorticoid treatment.
Figures
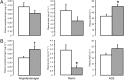
 , n = 5). Data are presented as mean values (±SEM). Significant difference from saline-treated group: *, P < .05; †, P = .06.
, n = 5). Data are presented as mean values (±SEM). Significant difference from saline-treated group: *, P < .05; †, P = .06.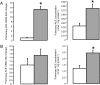
 , n = 5) treatment. Data are presented as mean values (±SEM); transcript data are presented as fold changes relative to the saline-treated group. Significant difference from saline-treated group: *, P < .05.
, n = 5) treatment. Data are presented as mean values (±SEM); transcript data are presented as fold changes relative to the saline-treated group. Significant difference from saline-treated group: *, P < .05.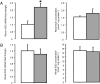
 , n = 5) treatment. Data are presented as mean values (±SEM); transcript data are presented as fold changes relative to the saline-treated group. Significant difference from saline-treated group: *, P < .05.
, n = 5) treatment. Data are presented as mean values (±SEM); transcript data are presented as fold changes relative to the saline-treated group. Significant difference from saline-treated group: *, P < .05.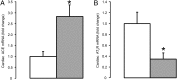
 , n = 5) treatment. Data are presented as mean fold changes (±SEM) relative to the saline-treated group. Significant difference from saline-treated group: *, P < .05.
, n = 5) treatment. Data are presented as mean fold changes (±SEM) relative to the saline-treated group. Significant difference from saline-treated group: *, P < .05.References
-
- Ballard PL, Ballard RA. Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am J Obstet Gynecol. 1995;173:254–262. - PubMed
-
- Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;CD004454. - PubMed
-
- Fowden AL, Li J, Forhead AJ. Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance? Proc Nutr Soc. 1998;57:113–122. - PubMed
-
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–525. - PubMed
-
- French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol. 1999;180:114–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

