Characterization of a Large Panel of Rabbit Monoclonal Antibodies against HIV-1 gp120 and Isolation of Novel Neutralizing Antibodies against the V3 Loop
- PMID: 26039641
- PMCID: PMC4454676
- DOI: 10.1371/journal.pone.0128823
Characterization of a Large Panel of Rabbit Monoclonal Antibodies against HIV-1 gp120 and Isolation of Novel Neutralizing Antibodies against the V3 Loop
Abstract
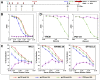
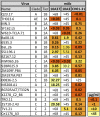
We recently reported the induction of potent, cross-clade neutralizing antibodies (nAbs) against Human Immunodeficiency Virus type-1 (HIV-1) in rabbits using gp120 based on an M-group consensus sequence. To better characterize these antibodies, 93 hybridomas were generated, which represent the largest panel of monoclonal antibodies (mAbs) ever generated from a vaccinated rabbit. The single most frequently recognized epitope of the isolated mAbs was at the very C-terminal end of the protein (APTKAKRRVVEREKR), followed by the V3 loop. A total of seven anti-V3 loop mAbs were isolated, two of which (10A3 and 10A37) exhibited neutralizing activity. In contrast to 10A3 and most other anti-V3 loop nAbs, 10A37 was atypical with its epitope positioned more towards the C-terminal half of the loop. To our knowledge, 10A37 is the most potent and broadly neutralizing anti-V3 loop mAb induced by vaccination. Interestingly, all seven anti-V3 loop mAbs competed with PGT121, suggesting a possibility that early induction of potent anti-V3 loop antibodies could prevent induction of more broadly neutralizing PGT121-like antibodies that target the conserved base of the V3 loop stem.
Conflict of interest statement
Figures






References
-
- Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature's pathways. Immunol Rev [Internet]. 2013. June 16;254(1):225–44. Available: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id... 10.1111/imr.12075 - DOI - PMC - PubMed
-
- Haynes BF, Moody MA, Alam M, Bonsignori M, Verkoczy L, Ferrari G, et al. Progress in HIV-1 vaccine development. Journal of Allergy and Clinical Immunology [Internet]. Elsevier Ltd; 2014. July 1;134(1):3–10. Available: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id... - PMC - PubMed
-
- Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, et al. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004. March;5(3):233–6. - PubMed
-
- Haynes BF, Montefiori DC. Aiming to induce broadly reactive neutralizing antibody responses with HIV-1 vaccine candidates. Expert Rev Vaccines. 2006. August;5(4):579–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

