Population pharmacokinetics of oseltamivir in non-pregnant and pregnant women
- PMID: 26040405
- PMCID: PMC4631177
- DOI: 10.1111/bcp.12691
Population pharmacokinetics of oseltamivir in non-pregnant and pregnant women
Abstract
Aims: Physiological changes in pregnancy are expected to alter the pharmacokinetics of various drugs. The objective of this study was to evaluate systematically the pharmacokinetics of oseltamivir (OS), a drug used in the treatment of influenza during pregnancy.
Methods: A multicentre steady-state pharmacokinetic study of OS was performed in 35 non-pregnant and 29 pregnant women. Plasma concentration-time profiles were analyzed using both non-compartmental and population pharmacokinetic modelling (pop PK) and simulation approaches. A one compartment population pharmacokinetic model with first order absorption and elimination adequately described the pharmacokinetics of OS.
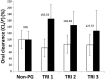
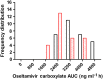
Results: The systemic exposure of oseltamivir carboxylate (OC, active metabolite of OS) was reduced approximately 30 (19-36)% (P < 0.001) in pregnant women. Pregnancy significantly (P < 0.001) influenced the clearance (CL/F) and volume of distribution (V/F) of OC. Both non-compartmental and population pharmacokinetic approaches documented approximately 45 (23-62)% increase in clearance (CL/F) of OC during pregnancy.
Conclusion: Based on the decrease in exposure of the active metabolite, the currently recommended doses of OS may need to be increased modestly in pregnant women in order to achieve comparable exposure with that of non-pregnant women.
Keywords: oseltamivir; population pharmacokinetics; pregnancy.
© 2015 The British Pharmacological Society.
Figures
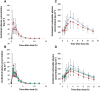
 non-pregnant,
non-pregnant,  pregnant. B and D
pregnant. B and D  non-pregnant,
non-pregnant,  trimester 1,
trimester 1,  trimester 2,
trimester 2,  trimester 3
trimester 3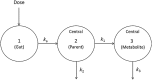
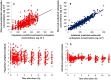
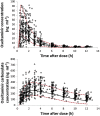
 5th and 95th percentile,
5th and 95th percentile,  50 percentile (median),
50 percentile (median),  Observants.
Observants.References
-
- Freeman DW, Barno A. Deaths from Asian influenza associated with pregnancy. Am J Obstet Gynecol. 1959;78:1172–5. - PubMed
-
- Mosby LG, Rasmussen SA, Jamieson DJ. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol. 2011;205:10–8. - PubMed
-
- Centers for Disease Control and Prevention. Available at http://www.cdc.gov/flu/protect/vaccine/pregnant.htm (last accessed 2014). In, Atlanta, GA, 2014.
-
- Cervantes-Gonzalez M, Launay O. Pandemic influenza A (H1N1) in pregnant women: impact of early diagnosis and antiviral treatment. Expert Rev Anti Infect Ther. 2010;8:981–4. - PubMed
-
- Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM. Antiviral agents for the treatment and chemoprophylaxis of influenza --- recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recommendations and reports: morbidity and mortality weekly report and reports / Centers for Disease Control. 2011;60:1–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

