Applications of the CRISPR-Cas9 system in cancer biology
- PMID: 26040603
- PMCID: PMC4530801
- DOI: 10.1038/nrc3950
Applications of the CRISPR-Cas9 system in cancer biology
Abstract
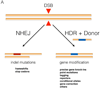
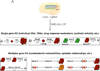
The prokaryotic type II CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats-CRISPR-associated 9) system is rapidly revolutionizing the field of genetic engineering, allowing researchers to alter the genomes of a large range of organisms with relative ease. Experimental approaches based on this versatile technology have the potential to transform the field of cancer genetics. Here, we review current approaches for functional studies of cancer genes that are based on CRISPR-Cas, with emphasis on their applicability for the development of next-generation models of human cancer.
Figures







References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Smithies O, Gregg RG, Boggs SS, Koralewski MA, Kucherlapati RS. Insertion of DNA sequences into the human chromosomal β-globin locus by homologous recombination. Nature. 1985;317:230–234. - PubMed
-
- Thomas KR, Folger KR, Capecchi MR. High frequency targeting of genes to specific sites in the mammalian genome. Cell. 1986;44:419–428. - PubMed
-
- Mansour SL, Thomas KR, Capecchi MR. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988;336:348–352. - PubMed
-
- Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7:645–658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

