Age and amyloid effects on human central nervous system amyloid-beta kinetics
- PMID: 26040676
- PMCID: PMC4546566
- DOI: 10.1002/ana.24454
Age and amyloid effects on human central nervous system amyloid-beta kinetics
Abstract
Objective: Age is the single greatest risk factor for Alzheimer's disease (AD), with the incidence doubling every 5 years after age 65. However, our understanding of the mechanistic relationship between increasing age and the risk for AD is currently limited. We therefore sought to determine the relationship between age, amyloidosis, and amyloid-beta (Aβ) kinetics in the central nervous system (CNS) of humans.
Methods: Aβ kinetics were analyzed in 112 participants and compared to the ages of participants and the amount of amyloid deposition.
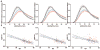
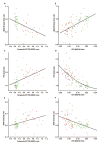
Results: We found a highly significant correlation between increasing age and slowed Aβ turnover rates (2.5-fold longer half-life over five decades of age). In addition, we found independent effects on Aβ42 kinetics specifically in participants with amyloid deposition. Amyloidosis was associated with a higher (>50%) irreversible loss of soluble Aβ42 and a 10-fold higher Aβ42 reversible exchange rate.
Interpretation: These findings reveal a mechanistic link between human aging and the risk of amyloidosis, which may be owing to a dramatic slowing of Aβ turnover, increasing the likelihood of protein misfolding that leads to deposition. Alterations in Aβ kinetics associated with aging and amyloidosis suggest opportunities for diagnostic and therapeutic strategies. More generally, this study provides an example of how changes in protein turnover kinetics can be used to detect physiological and pathophysiological changes and may be applicable to other proteinopathies.
© 2015 American Neurological Association.
Conflict of interest statement
Drs. Bateman, Holtzman and Patterson report personal fees for consulting outside the submitted work from C2N Diagnostics, which has licensed related patents from Washington University. The following authors are inventors on patents held by Washington University in which they are eligible for personal fees and/or fees to their laboratories: RJB, BWP, DLE, KGW, DMH. Mr. Ovod, Ms. Ma, Mr. Chott, Dr. Yarasheski, Mrs. Sigurdson, and Ms. Zhang have nothing to disclose.
Figures





References
-
- Thies W, Bleiler L, Alzheimer’s A. 2013 Alzheimer’s disease facts and figures Alzheimer’s Association. Alzheimers & Dementia. 2013 Mar;9(2):208–45. - PubMed
-
- Jorm AF, Jolley D. The incidence of dementia - A meta-analysis. Neurology. 1998 Sep;51(3):728–33. - PubMed
-
- Roses AD, Saunders AM. APOE is a major susceptibility gene for Alzheimer’s disease. Current opinion in biotechnology 1994. 1994 Dec;5(6):663–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AG05681/AG/NIA NIH HHS/United States
- R01NS065667/NS/NINDS NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- R01 NS065667/NS/NINDS NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- P41 GM103422/GM/NIGMS NIH HHS/United States
- P01GM103422/GM/NIGMS NIH HHS/United States
- K23 AG030946/AG/NIA NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- K23AG030946/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

