Role of nitric oxide in murine conventional outflow physiology
- PMID: 26040898
- PMCID: PMC4537932
- DOI: 10.1152/ajpcell.00347.2014
Role of nitric oxide in murine conventional outflow physiology
Abstract
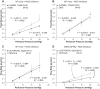
Elevated intraocular pressure (IOP) is the main risk factor for glaucoma. Exogenous nitric oxide (NO) decreases IOP by increasing outflow facility, but whether endogenous NO production contributes to the physiological regulation of outflow facility is unclear. Outflow facility was measured by pressure-controlled perfusion in ex vivo eyes from C57BL/6 wild-type (WT) or transgenic mice expressing human endothelial NO synthase (eNOS) fused to green fluorescent protein (GFP) superimposed on the endogenously expressed murine eNOS (eNOS-GFPtg). In WT mice, exogenous NO delivered by 100 μM S-nitroso-N-acetylpenicillamine (SNAP) increased outflow facility by 62 ± 28% (SD) relative to control eyes perfused with the inactive SNAP analog N-acetyl-d-penicillamine (NAP; n = 5, P = 0.016). In contrast, in eyes from eNOS-GFPtg mice, SNAP had no effect on outflow facility relative to NAP (-9 ± 4%, P = 0.40). In WT mice, the nonselective NOS inhibitor N(G)-nitro-l-arginine methyl ester (l-NAME, 10 μM) decreased outflow facility by 36 ± 13% (n = 5 each, P = 0.012), but 100 μM l-NAME had no detectable effect on outflow facility (-16 ± 5%, P = 0.22). An eNOS-selective inhibitor (cavtratin, 50 μM) decreased outflow facility by 19 ± 12% in WT (P = 0.011) and 39 ± 25% in eNOS-GFPtg (P = 0.014) mice. In the conventional outflow pathway of eNOS-GFPtg mice, eNOS-GFP expression was localized to endothelial cells lining Schlemm's canal and the downstream vessels, with no apparent expression in the trabecular meshwork. These results suggest that endogenous NO production by eNOS within endothelial cells of Schlemm's canal or downstream vessels contributes to the physiological regulation of aqueous humor outflow facility in mice, representing a viable strategy to more successfully lower IOP in glaucoma.
Keywords: aqueous humor outflow; glaucoma; intraocular pressure; mouse model; nitric oxide.
Copyright © 2015 the American Physiological Society.
Figures




Comment in
-
Seeing is believing: NO therapy for glaucoma? Focus on "Role of nitric oxide in murine conventional outflow physiology".Am J Physiol Cell Physiol. 2015 Aug 15;309(4):C203-4. doi: 10.1152/ajpcell.00180.2015. Epub 2015 Jun 24. Am J Physiol Cell Physiol. 2015. PMID: 26108667 Free PMC article. No abstract available.
References
-
- Alvarado JA, Katz LJ, Trivedi S, Shifera AS. Monocyte modulation of aqueous outflow and recruitment to the trabecular meshwork following selective laser trabeculoplasty. Arch Ophthalmol 128: 731–737, 2010. - PubMed
-
- Bachmann B, Birke M, Kook D, Eichhorn M, Lütjen-Drecoll E. Ultrastructural and biochemical evaluation of the porcine anterior chamber perfusion model. Invest Ophthalmol Vis Sci 47: 2011–2020, 2006. - PubMed
-
- Barany E. The mode of action of pilocarpine on outflow resistance in the eye of a primate (Cercopithecus ethiops). Invest Ophthalmol 1: 712–727, 1962. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

