Poxvirus Protein MC132 from Molluscum Contagiosum Virus Inhibits NF-B Activation by Targeting p65 for Degradation
- PMID: 26041281
- PMCID: PMC4524246
- DOI: 10.1128/JVI.00799-15
Poxvirus Protein MC132 from Molluscum Contagiosum Virus Inhibits NF-B Activation by Targeting p65 for Degradation
Abstract
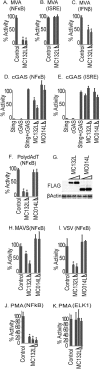
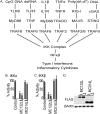
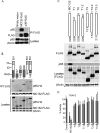
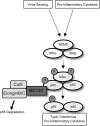
Molluscum contagiosum virus (MCV) is unique in being the only known extant, human-adapted poxvirus, yet to date, it is very poorly characterized in terms of host-pathogen interactions. MCV causes persistent skin lesions filled with live virus, but these are generally immunologically silent, suggesting the presence of potent inhibitors of human antiviral immunity and inflammation. Fewer than five MCV immunomodulatory genes have been characterized in detail, but it is likely that many more remain to be discovered given the density of such sequences in all well-characterized poxviruses. Following virus infection, NF-B activation occurs in response to both pattern recognition receptor (PRR) signaling and cellular activation by virus-elicited proinflammatory cytokines, such as tumor necrosis factor (TNF). As such, NF-B activation is required for virus detection, antiviral signaling, inflammation, and clearance of viral infection. Hence, we screened a library of MCV genes for effects on TNF-stimulated NF-B activation. This revealed MC132, a unique protein with no orthologs in other poxviral genomes, as a novel inhibitor of NF-B. Interestingly, MC132 also inhibited PRR- and virus-activated NF-B, since MC132 interacted with the NF-B subunit p65 and caused p65 degradation. Unbiased affinity purification to identify host targets of MC132 revealed that MC132 acted by targeting NF-B p65 for ubiquitin-dependent proteasomal degradation by recruiting p65 to a host Cullin-5/Elongin B/Elongin C complex. These data reveal a novel mechanism for poxviral inhibition of human innate immunity and further clarify how the human-adapted poxvirus MCV can so effectively evade antiviral immunity to persist in skin lesions.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

