Mapping of a Region of the PA-X Protein of Influenza A Virus That Is Important for Its Shutoff Activity
- PMID: 26041295
- PMCID: PMC4524237
- DOI: 10.1128/JVI.01132-15
Mapping of a Region of the PA-X Protein of Influenza A Virus That Is Important for Its Shutoff Activity
Abstract
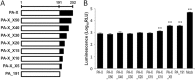
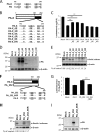
Influenza A virus PA-X comprises an N-terminal PA endonuclease domain and a C-terminal PA-X-specific domain. PA-X reduces host and viral mRNA accumulation via its endonuclease function. Here, we found that the N-terminal 15 amino acids, particularly six basic amino acids, in the C-terminal PA-X-specific region are important for PA-X shutoff activity. These six basic amino acids enabled a PA deletion mutant to suppress protein expression at a level comparable to that of wild-type PA-X.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Jagger BW, Wise HM, Kash JC, Walters KA, Wills NM, Xiao YL, Dunfee RL, Schwartzman LM, Ozinsky A, Bell GL, Dalton RM, Lo A, Efstathiou S, Atkins JF, Firth AE, Taubenberger JK, Digard P. 2012. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science 337:199–204. doi:10.1126/science.1222213. - DOI - PMC - PubMed
-
- Gao H, Sun H, Hu J, Qi L, Wang J, Xiong X, Wang Y, He Q, Lin Y, Kong W, Seng LG, Pu J, Chang KC, Liu X, Liu J, Sun Y. 15 April 2015. The 20 amino acids at the C-terminus of PA-X are associated with increased influenza A virus replication and pathogenicity. J Gen Virol doi:10.1099/vir.0.000143. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

