Broadly Neutralizing Human Immunodeficiency Virus Type 1 Antibody Gene Transfer Protects Nonhuman Primates from Mucosal Simian-Human Immunodeficiency Virus Infection
- PMID: 26041300
- PMCID: PMC4524228
- DOI: 10.1128/JVI.00908-15
Broadly Neutralizing Human Immunodeficiency Virus Type 1 Antibody Gene Transfer Protects Nonhuman Primates from Mucosal Simian-Human Immunodeficiency Virus Infection
Abstract
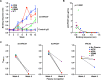
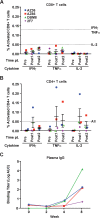
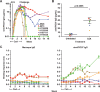
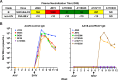
Broadly neutralizing antibodies (bnAbs) can prevent lentiviral infection in nonhuman primates and may slow the spread of human immunodeficiency virus type 1 (HIV-1). Although protection by passive transfer of human bnAbs has been demonstrated in monkeys, durable expression is essential for its broader use in humans. Gene-based expression of bnAbs provides a potential solution to this problem, although immune responses to the viral vector or to the antibody may limit its durability and efficacy. Here, we delivered an adeno-associated viral vector encoding a simianized form of a CD4bs bnAb, VRC07, and evaluated its immunogenicity and protective efficacy. The expressed antibody circulated in macaques for 16 weeks at levels up to 66 g/ml, although immune suppression with cyclosporine (CsA) was needed to sustain expression. Gene-delivered simian VRC07 protected against simian-human immunodeficiency virus (SHIV) infection in monkeys 5.5 weeks after treatment. Gene transfer of an anti-HIV antibody can therefore protect against infection by viruses that cause AIDS in primates when the host immune responses are controlled.
Figures


References
-
- Doria-Rose NA, Klein RM, Manion MM, O'Dell S, Phogat A, Chakrabarti B, Hallahan CW, Migueles SA, Wrammert J, Ahmed R, Nason M, Wyatt RT, Mascola JR, Connors M. 2009. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J Virol 83:188–199. doi: 10.1128/JVI.01583-08. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

