CARMA3 Is Critical for the Initiation of Allergic Airway Inflammation
- PMID: 26041536
- PMCID: PMC4489191
- DOI: 10.4049/jimmunol.1402983
CARMA3 Is Critical for the Initiation of Allergic Airway Inflammation
Abstract
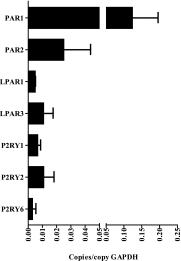
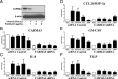
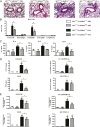
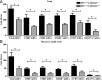
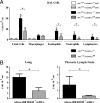
Innate immune responses to allergens by airway epithelial cells (AECs) help initiate and propagate the adaptive immune response associated with allergic airway inflammation in asthma. Activation of the transcription factor NF-κB in AECs by allergens or secondary mediators via G protein-coupled receptors (GPCRs) is an important component of this multifaceted inflammatory cascade. Members of the caspase recruitment domain family of proteins display tissue-specific expression and help mediate NF-κB activity in response to numerous stimuli. We have previously shown that caspase recruitment domain-containing membrane-associated guanylate kinase protein (CARMA)3 is specifically expressed in AECs and mediates NF-κB activation in these cells in response to stimulation with the GPCR agonist lysophosphatidic acid. In this study, we demonstrate that reduced levels of CARMA3 in normal human bronchial epithelial cells decreases the production of proasthmatic mediators in response to a panel of asthma-relevant GPCR ligands such as lysophosphatidic acid, adenosine triphosphate, and allergens that activate GPCRs such as Alternaria alternata and house dust mite. We then show that genetically modified mice with CARMA3-deficient AECs have reduced airway eosinophilia and proinflammatory cytokine production in a murine model of allergic airway inflammation. Additionally, we demonstrate that these mice have impaired dendritic cell maturation in the lung and that dendritic cells from mice with CARMA3-deficient AECs have impaired Ag processing. In conclusion, we show that AEC CARMA3 helps mediate allergic airway inflammation, and that CARMA3 is a critical signaling molecule bridging the innate and adaptive immune responses in the lung.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures


References
-
- Busse W. W., Lemanske R. F., Jr. 2001. Asthma. N. Engl. J. Med. 344: 350–362. - PubMed
-
- Mellman I., Steinman R. M. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell 106: 255–258. - PubMed
-
- Huh J. C., Strickland D. H., Jahnsen F. L., Turner D. J., Thomas J. A., Napoli S., Tobagus I., Stumbles P. A., Sly P. D., Holt P. G. 2003. Bidirectional interactions between antigen-bearing respiratory tract dendritic cells (DCs) and T cells precede the late phase reaction in experimental asthma: DC activation occurs in the airway mucosa but not in the lung parenchyma. J. Exp. Med. 198: 19–30. - PMC - PubMed
-
- Holt P. G., Schon-Hegrad M. A., Oliver J., Holt B. J., McMenamin P. G. 1990. A contiguous network of dendritic antigen-presenting cells within the respiratory epithelium. Int. Arch. Allergy Appl. Immunol. 91: 155–159. - PubMed
-
- de Jong E. C., Smits H. H., Kapsenberg M. L. 2005. Dendritic cell-mediated T cell polarization. Springer Semin. Immunopathol. 26: 289–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

