DICER/AGO-dependent epigenetic silencing of D4Z4 repeats enhanced by exogenous siRNA suggests mechanisms and therapies for FSHD
- PMID: 26041815
- PMCID: PMC4527486
- DOI: 10.1093/hmg/ddv206
DICER/AGO-dependent epigenetic silencing of D4Z4 repeats enhanced by exogenous siRNA suggests mechanisms and therapies for FSHD
Abstract
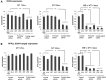
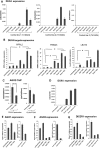
Facioscapulohumeral muscular dystrophy (FSHD) is caused by the aberrant expression of the DUX4 transcription factor in skeletal muscle. The DUX4 retrogene is encoded in the D4Z4 macrosatellite repeat array, and smaller array size or a mutation in the SMCHD1 gene results in inefficient epigenetic repression of DUX4 in skeletal muscle, causing FSHD1 and FSHD2, respectively. Previously we showed that the entire D4Z4 repeat is bi-directionally transcribed with the generation of small si- or miRNA-like fragments and suggested that these might suppress DUX4 expression through the endogenous RNAi pathway. Here we show that exogenous siRNA targeting the region upstream of the DUX4 transcription start site suppressed DUX4 mRNA expression and increased both H3K9 methylation and AGO2 recruitment. In contrast, similarly targeted MOE-gapmer antisense oligonucleotides that degrade RNA but do not engage the RNAi pathway did not repress DUX4 expression. In addition, knockdown of DICER or AGO2 using either siRNA or MOE-gapmer chemistries resulted in the induction of DUX4 expression in control muscle cells that normally do not express DUX4, indicating that the endogenous RNAi pathway is necessary to maintain repression of DUX4 in control muscle cells. Together these data demonstrate a role of the endogenous RNAi pathway in repeat-mediated epigenetic repression of the D4Z4 macrosatellite repeat, and show that enhancing the activity of this pathway by supplying exogenous siRNA oligonucleotides represents a potential therapeutic approach to silencing DUX4 in FSHD.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Lemmers R.J., Tawil R., Petek L.M., Balog J., Block G.J., Santen G.W., Amell A.M., van der Vliet P.J., Almomani R., Straasheijm K.R. et al. (2012) Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes facioscapulohumeral muscular dystrophy type 2. Nat. Genet., 44, 1370–1374. - PMC - PubMed
-
- Blewitt M.E., Gendrel A.V., Pang Z., Sparrow D.B., Whitelaw N., Craig J.M., Apedaile A., Hilton D.J., Dunwoodie S.L., Brockdorff N. et al. (2008) SmcHD1, containing a structural-maintenance-of-chromosomes hinge domain, has a critical role in X inactivation. Nat. Genet., 40, 663–669. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

