Lentiviral vectors as tools to understand central nervous system biology in mammalian model organisms
- PMID: 26041987
- PMCID: PMC4434958
- DOI: 10.3389/fnmol.2015.00014
Lentiviral vectors as tools to understand central nervous system biology in mammalian model organisms
Abstract
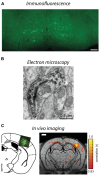
Lentiviruses have been extensively used as gene delivery vectors since the mid-1990s. Usually derived from the human immunodeficiency virus genome, they mediate efficient gene transfer to non-dividing cells, including neurons and glia in the adult mammalian brain. In addition, integration of the recombinant lentiviral construct into the host genome provides permanent expression, including the progeny of dividing neural precursors. In this review, we describe targeted vectors with modified envelope glycoproteins and expression of transgenes under the regulation of cell-selective and inducible promoters. This technology has broad utility to address fundamental questions in neuroscience and we outline how this has been used in rodents and primates. Combining viral tract tracing with immunohistochemistry and confocal or electron microscopy, lentiviral vectors provide a tool to selectively label and trace specific neuronal populations at gross or ultrastructural levels. Additionally, new generation optogenetic technologies can be readily utilized to analyze neuronal circuit and gene functions in the mature mammalian brain. Examples of these applications, limitations of current systems and prospects for future developments to enhance neuroscience knowledge will be reviewed. Finally, we will discuss how these vectors may be translated from gene therapy trials into the clinical setting.
Keywords: confocal and electron microscopy; lentivirus; neuron phenotype; optogenetics; temporal and spatial specificity.
Figures
References
-
- Annoni A., Battaglia M., Follenzi A., Lombardo A., Sergi-Sergi L., Naldini L., et al. (2007). The immune response to lentiviral-delivered transgene is modulated in vivo by transgene-expressing antigen-presenting cells but not by CD4+CD25+ regulatory T cells. Blood 110 1788–1796 10.1182/blood-2006-11-059873 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

