Altered neuronal excitability underlies impaired hippocampal function in an animal model of psychosis
- PMID: 26042007
- PMCID: PMC4438226
- DOI: 10.3389/fnbeh.2015.00117
Altered neuronal excitability underlies impaired hippocampal function in an animal model of psychosis
Abstract
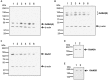
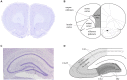
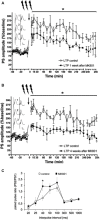
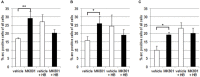
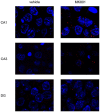
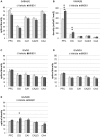
Psychosis is accompanied by severe attentional deficits, and impairments in associational-memory processing and sensory information processing that are ascribed to dysfunctions in prefrontal and hippocampal function. Disruptions of glutamatergic signaling may underlie these alterations: Antagonism of the N-methyl-D-aspartate receptor (NMDAR) results in similar molecular, cellular, cognitive and behavioral changes in rodents and/or humans as those that occur in psychosis, raising the question as to whether changes in glutamatergic transmission may be intrinsic to the pathophysiology of the disease. In an animal model of psychosis that comprises treatment with the irreversible NMDAR-antagonist, MK801, we explored the cellular mechanisms that may underlie hippocampal dysfunction in psychosis. MK801-treatment resulted in a profound loss of hippocampal LTP that was evident 4 weeks after treatment. Whereas neuronal expression of the immediate early gene, Arc, was enhanced in the hippocampus by spatial learning in controls, MK801-treated animals failed to show activity-dependent increases in Arc expression. By contrast, a significant increase in basal Arc expression in the absence of learning was evident compared to controls. Paired-pulse (PP) facilitation was increased at the 40 ms interval indicating that NMDAR and/or fast GABAergic-mediated neurotransmission was disrupted. In line with this, MK801-treatment resulted in a significant decrease in GABA(A), and increase in GABA(B)-receptor-expression in PFC, along with a significant increase of GABA(B)- and NMDAR-GluN2B expression in the dentate gyrus. NMDAR-GluN1 or GluN2A subunit expression was unchanged. These data suggest that in psychosis, deficits in hippocampus-dependent memory may be caused by a loss of hippocampal LTP that arises through enhanced hippocampal neuronal excitability, altered GluN2B and GABA receptor expression and an uncoupling of the hippocampus-prefrontal cortex circuitry.
Keywords: GABA; MK801; NMDA receptor hypofunction; hippocampus; in vivo; schizophrenia.
Figures

References
-
- Álvarez-Jiménez M., Gleeson J. F., Henry L. P., Harrigan S. M., Harris M. G., Killackey E., et al. (2012). Road to full recovery, longitudinal relationship between symptomatic remission and psychosocial recovery in first-episode psychosis over 7.5 years. Psychol. Med. 42, 595–606. 10.1017/s0033291711001504 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

