3D Printed Programmable Release Capsules
- PMID: 26042472
- PMCID: PMC4536147
- DOI: 10.1021/acs.nanolett.5b01688
3D Printed Programmable Release Capsules
Abstract
The development of methods for achieving precise spatiotemporal control over chemical and biomolecular gradients could enable significant advances in areas such as synthetic tissue engineering, biotic-abiotic interfaces, and bionanotechnology. Living organisms guide tissue development through highly orchestrated gradients of biomolecules that direct cell growth, migration, and differentiation. While numerous methods have been developed to manipulate and implement biomolecular gradients, integrating gradients into multiplexed, three-dimensional (3D) matrices remains a critical challenge. Here we present a method to 3D print stimuli-responsive core/shell capsules for programmable release of multiplexed gradients within hydrogel matrices. These capsules are composed of an aqueous core, which can be formulated to maintain the activity of payload biomolecules, and a poly(lactic-co-glycolic) acid (PLGA, an FDA approved polymer) shell. Importantly, the shell can be loaded with plasmonic gold nanorods (AuNRs), which permits selective rupturing of the capsule when irradiated with a laser wavelength specifically determined by the lengths of the nanorods. This precise control over space, time, and selectivity allows for the ability to pattern 2D and 3D multiplexed arrays of enzyme-loaded capsules along with tunable laser-triggered rupture and release of active enzymes into a hydrogel ambient. The advantages of this 3D printing-based method include (1) highly monodisperse capsules, (2) efficient encapsulation of biomolecular payloads, (3) precise spatial patterning of capsule arrays, (4) "on the fly" programmable reconfiguration of gradients, and (5) versatility for incorporation in hierarchical architectures. Indeed, 3D printing of programmable release capsules may represent a powerful new tool to enable spatiotemporal control over biomolecular gradients.
Keywords: 3D printing; biomolecular gradients; core−shell particles; plasmonic nanorods; release capsules; spatiotemporal patterning.
Figures
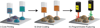

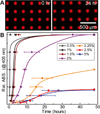
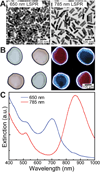
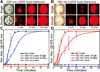

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

