Cyclo-oxygenase (COX) inhibitors for treating preterm labour
- PMID: 26042617
- PMCID: PMC7068172
- DOI: 10.1002/14651858.CD001992.pub3
Cyclo-oxygenase (COX) inhibitors for treating preterm labour
Abstract
Background: Preterm birth is a major cause of perinatal mortality and morbidity. Cyclo-oxygenase (COX) inhibitors inhibit uterine contractions, are easily administered and appear to have few maternal side effects. However, adverse effects have been reported in the fetus and newborn as a result of exposure to COX inhibitors.
Objectives: To assess the effects on maternal and neonatal outcomes of COX inhibitors administered as a tocolytic agent to women in preterm labour when compared with (i) placebo or no intervention and (ii) other tocolytics. In addition, to compare the effects of non-selective COX inhibitors with COX-2 selective inhibitors.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (24 August 2014). We also contacted recognised experts and searched reference lists of retrieved studies.
Selection criteria: All published and unpublished randomised trials in which COX inhibitors were used for tocolysis for women in labour between 20 and 36 completed weeks' gestation.
Data collection and analysis: Two review authors independently evaluated methodological quality and extracted data. We sought additional information from study authors. Results are presented using risk ratio (RR; dichotomous data) and mean difference (MD; continuous data) with 95% confidence interval (CI). The number needed to treat for benefit (NNTB) and the number needed to treat for harm (NNTH) were calculated for statistically different categorical outcomes.
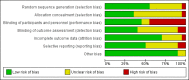
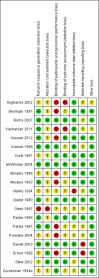
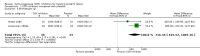
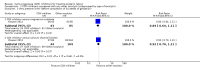
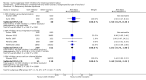
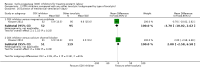
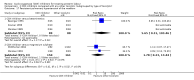
Main results: With the addition of seven studies with a total of 684 women, this review now includes outcome data from 20 studies including 1509 women. The non-selective COX inhibitor indomethacin was used in 15 studies. The overall quality of the included studies was considered moderate to low.Three small studies (102 women), two of which were conducted in the 1980's, compared COX inhibition (indomethacin only) with placebo. No difference was shown in birth less than 48 hours after trial entry (average RR 0.20, 95% CI 0.03 to 1.28; two studies with 70 women). Indomethacin resulted in a reduction in preterm birth (before completion of 37 weeks of gestation) in one small study (36 women) (RR 0.21, 95% CI 0.07 to 0.62; NNTB 2, 95% CI 2 to 4); and an increase in gestational age at birth (average MD 3.59 weeks, 95% CI 0.65 to 6.52; two studies with 66 women) and birthweight (MD 716.34 g, 95% CI 425.52 to 1007.16; two studies with 67 infants). No difference was shown in measures of neonatal morbidity or neonatal mortality.Compared with betamimetics, COX inhibitors resulted in a reduction in birth less than 48 hours after trial entry (RR 0.27, 95% CI 0.08 to 0.96; NNTB 7, 95% CI 6 to 120; two studies with 100 women) and preterm birth (before completion of 37 weeks of gestation) (RR 0.53, 95% CI 0.28 to 0.99; NNTB 6, 95% CI 4 to 236; two studies with 80 women) although no benefit was shown in terms of neonatal morbidity or mortality. COX inhibition was also associated with fewer maternal adverse affects compared with betamimetics (RR 0.19, 95% CI 0.11 to 0.31; NNTB 3, 95% CI 2 to 3; five studies with 248 women) and maternal adverse effects requiring cessation of treatment (average RR 0.09, 95% CI 0.02 to 0.49; NNTB 5, CI 95% 5 to 9; three studies with 166 women).No differences were shown when comparing COX inhibitors with magnesium sulphate (MgSO4) (seven studies with 792 women) or calcium channel blockers (CCBs) (two studies with 230 women) in terms of prolonging pregnancy or for any fetal/neonatal outcomes. There were also no differences in very preterm birth (before completion of 34 weeks of gestation) and no maternal deaths occurred in the one study that reported on this outcome. However COX inhibitors resulted in fewer maternal adverse affects when compared with MgSO4 (RR 0.39, 95% CI 0.25 to 0.62; NNTB 11, 95% CI 9 to 17; five studies with 635 women).A comparison of non-selective COX inhibitors versus any COX-2 inhibitor (two studies with 54 women) did not demonstrate any differences in maternal, fetal or neonatal outcomes.No data were available to assess COX inhibitors compared with oxytocin receptor antagonists (ORAs). Further, no data were available on extremely preterm birth (before 28 weeks of gestation), longer-term infant outcomes or costs.
Authors' conclusions: In this review, no clear benefit for COX inhibitors was shown over placebo or any other tocolytic agents. While some benefit was demonstrated in terms of postponement of birth for COX inhibitors over placebo and betamimetics and also maternal adverse effects over betamimetics and MgSO4, due to the limitations of small numbers, minimal data on safety, lack of longer-term outcomes and generally low quality of the studies included in this review, we conclude that there is insufficient evidence on which to base decisions about the role of COX inhibition for women in preterm labour. Further well-designed tocolytic studies are required to determine short- and longer-term infant benefit of COX inhibitors over placebo and other tocolytics, particularly CCBs and ORAs. Another important focus for future studies is identifying whether COX-2 inhibitors are superior to non-selective COX inhibitors. All future studies on tocolytics for women in preterm labour should assess longer-term effects into early childhood and also costs.
Conflict of interest statement
All authors declared no conflict of interest.
Figures


































































Update of
-
Cyclo-oxygenase (COX) inhibitors for treating preterm labour.Cochrane Database Syst Rev. 2005 Apr 18;(2):CD001992. doi: 10.1002/14651858.CD001992.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2015 Jun 05;(6):CD001992. doi: 10.1002/14651858.CD001992.pub3. PMID: 15846626 Updated.
References
References to studies included in this review
Asgharnia 2002 {published data only}
-
- Asgharnia M, Sobhani A, Omidvar‐Jalali Z. Comparison of Mg‐Sulfate and indomethacin in management of women with preterm labor. Journal of Gorgan University of Medical Sciences 2002;4(2):7‐12.
Besinger 1991 {published data only}
-
- Besinger RE, Niebyl JR, Keyes WG, Johnson TR. Randomized comparative trial of indomethacin and ritodrine for the long‐term treatment of preterm labor. American Journal of Obstetrics and Gynecology 1991;164(4):981‐8. - PubMed
Borna 2007 {published data only}
-
- Borna S, Saeidi FM. Celecoxib versus magnesium sulfate to arrest preterm labor: randomized trial. Journal of Obstetrics and Gynaecology Research 2007;33(5):631‐4. - PubMed
Kashanian 2011 {published data only}
-
- Kashanian M, Bahasadri S, Zolali B. Comparison of the efficacy and adverse effects of nifedipine and indomethacin for the treatment of preterm labor. International Journal of Gynecology & Obstetrics 2011;113(3):192‐5. - PubMed
Klauser 2012 {published data only}
-
- Klauser CK, Briery CM, Keiser SD, Martin RW, Kosek MA, Morrison JC. Effect of antenatal tocolysis on neonatal outcomes. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(12):2778‐81. - PubMed
-
- Klauser CK, Briery CM, Martin RW, Langston L, Magann EF, Morrison JC. A comparison of three tocolytics for preterm labor: a randomized clinical trial. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(8):801‐6. - PubMed
Kramer 1999 {published data only}
-
- Kramer W, Saade G, Belfort M, Dorman K, Mayes MMK. Randomized double‐blind study comparing sulindac to terbutaline: fetal cardiovascular effects. American Journal of Obstetrics and Gynecology 1996;174(1 Pt 2):326. - PubMed
-
- Kramer WB, Saade GR, Belfort M, Dorman K, Mayes M, Moise KJ. A randomized double‐blind study comparing the fetal effects of sulindac to terbutaline during the management of preterm labor. American Journal of Obstetrics and Gynecology 1999;180(2):396‐401. - PubMed
Kurki 1991 {published data only}
-
- Eronen M. The hemodynamic effects of antenatal indomethacin in a beta‐sympathomimetic agent on the fetus and the newborn: a randomized study. Pediatric Research 1993;33(6):615‐9. - PubMed
-
- Eronen M, Pesonen E, Kurki T, Ylikorkala O, Hallman M. The effects of indomethacin and beta‐sympathomimetic agent on the fetal ductus arteriosus during treatment of premature labor: A randomized double‐blind study. American Journal of Obstetrics and Gynecology 1991;164:141‐6. - PubMed
-
- Eronen N, Pesonen E, Kurki T, Teramo K, Ylikorkala O, Hallman M. Increased incidence of bronchopulmonary dysplasia after antenatal administration of indomethacin to prevent preterm labor. Journal of Pediatrics 1994;124:782‐8. - PubMed
-
- Kurki T, Eronen M, Lumme R, Ylikorkala O. A randomized double‐dummy comparison between indomethacin and nylidrin in threatened preterm labor. Obstetrics & Gynecology 1991;78(6):1093‐7. - PubMed
-
- Kurki T, Laatikainen T, Salminen‐Lappalainen K, Ylikorkala O. Maternal plasma corticotrophin‐releasing hormone ‐ elevated in preterm labour but unaffected by indomethacin or nylidrin. British Journal of Obstetrics and Gynaecology 1991;98:685‐91. - PubMed
McWhorter 2004 {published data only}
-
- McWhorter J, Carlan S, O'Leary T, Richici K, O'Brien W. Rofecoxib versus magnesium sulfate to arrest preterm labour: a randomised trial. Obstetrics & Gynecology 2004;103:923‐30. - PubMed
-
- McWhorter J, Carlan SJ, O'Leary TD. Rofecoxib versus magnesium sulfate to arrest preterm labor: a randomized double‐blind trial. Obstetrics & Gynecology 2002;99(4 Suppl 1):2S. - PubMed
Morales 1989 {published data only}
-
- Morales WJ, Smith SG, Angel JL, O'Brien WF. Efficacy and safety of indomethacin vs ritodrine in the management of preterm labor: a randomized study. Proceedings of 9th Annual Meeting of the Society of Perinatal Obstetricians; 1989 Feb 1‐4; New Orleans, Louisiana, USA. 1989:35.
-
- Morales WJ, Smith SG, Angel JL, O'Brien WF, Knuppel RA. Efficacy and safety of indomethacin versus ritodrine in the management of preterm labor: a randomized study. Obstetrics & Gynecology 1989;74(4):567‐72. - PubMed
Morales 1993 {published data only}
-
- Morales WJ, Madhav H. Efficacy and safety of indomethacin compared with magnesium sulfate in the management of preterm labor: a randomized study. JAMA 1993;270:2676. - PubMed
-
- Morales WJ, Madhav H. Efficacy and safety of indomethacin compared with magnesium sulfate in the management or preterm labor: a randomized study. American Journal of Obstetrics and Gynecology 1993;169(1):97‐102. - PubMed
-
- Morales WJ, Madhav H. Efficacy and safety of indomethacin vs. magnesium in the management of preterm labor: a randomized study. American Journal of Obstetrics and Gynecology 1991;164:280. - PubMed
Nevils 1994 {published data only}
-
- Nevils B, Curet L, Izquierdo L, Chatterjee M, Gilson G, Maciulla J, et al. Indomethacin as a "rescue" tocolytic in pre‐term labor. American Journal of Obstetrics and Gynecology 1994;170:378. - PubMed
Niebyl 1980 {published data only}
-
- Blake DA, Niebyl JR, White RD, Kumor KM, Dubin NH, Robinson JC, et al. Treatment of premature labor with indomethacin. Advances in prostaglandin, thromboxane and leukotriene research 1980;8:1465‐7. - PubMed
-
- Niebyl JR, Blake DA, White RD, Kumor KM, Dubin NH, Robinson JC, et al. The inhibition of premature labor with indomethacin. American Journal of Obstetrics and Gynecology 1980;136(8):1014‐9. - PubMed
Odeh 1997 {published data only}
-
- Odeh M, Kaiis M, Markowitz J, Oettinger M. Progesterone levels in preterm labor are not affected by ritodrin treatment. Gynecologic and Obstetric Investigation 1997;43(1):34‐6. - PubMed
Panter 1999 {published data only}
-
- Cavalle‐Garrido T, Panter K, Smallhorn J, Seaward D, Farine D. A RCT of indomethacin for preterm labor: effects on fetal heart and ductus arteriosus. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S46.
-
- Panter K, Hannah M, Farine D, Amankwah K, Jefferies A, Ohlsson A. The effect of indomethacin tocolysis of preterm labor on perinatal outcome: a RCT. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S46. - PubMed
-
- Panter KR, Hannah ME, Amankwah KS, Ohlsson A, Jefferies AL, Farine D. The effect of indomethacin tocolysis in preterm labour on perinatal outcome: a randomised placebo‐controlled trial. British Journal of Obstetrics and Gynaecology 1999;106(5):467‐73. - PubMed
Parilla 1997 {published data only}
-
- Parilla BV, Tamura RK, Cohen LS, Clark E. Lack of effect of antenatal indomethacin on fetal cerebral blood flow. American Journal of Obstetrics and Gynecology 1997;176(6):1166‐71. - PubMed
Purwaka 2004 {published data only}
-
- Purwaka BT, Abadi A, Prasetyadi FOH. Comparison of efficacy between oral nimesulide and parenteral‐oral isoxsuprine to delay threatened preterm labour. Journal of Obstetrics and Gynaecology Research 2004;30(3):264.
Sawdy 2003 {published data only}
-
- Sawdy RJ, Lye S, Fisk NM, Bennett PR. A double‐blind randomized study of fetal side effects during and after the short‐term maternal administration of indomethacin, sulindac, and nimesulide for the treatment of preterm labor. American Journal of Obstetrics and Gynecology 2003;188:1046‐51. - PubMed
Schorr 1998 {published data only}
-
- Schorr SJ, Ascarelli MH, Rust OA, Ross EL, Calfee EL, Perry KG Jr, et al. A comparative study of ketorolac (Toradol) and magnesium sulfate for arrest of preterm labor. Southern Medical Journal 1998;91(11):1028‐32. - PubMed
-
- Schorr SJ, Ascarelli MH, Rust OA, Ross EL, Calfee EL, Perry KG Jr, et al. Ketorolac is a safe and effective drug for acute tocolysis. American Journal of Obstetrics and Gynecology 1997;176(1):S7.
Stika 2002 {published data only}
-
- Stika CS, Gross GA, Leguizamon G, Gerber S, Levy R, Mathur A, et al. A prospective randomized safety trial of celecoxib for treatment of preterm labor. American Journal of Obstetrics and Gynecology 2002;187(3):653‐60. - PubMed
Zuckerman 1984a {published data only}
-
- Zuckerman H, Shalev E, Gilad G, Katzuni E. Further study of the inhibition of premature labor by indomethacin. Part II double‐blind study. Journal of Perinatal Medicine 1984;12(1):25‐9. - PubMed
References to studies excluded from this review
Abramov 2000 {published data only}
-
- Abramov Y, Nadjari M, Weinstein D, Ben‐Shachar I, Plotkin V, Ezra Y. Indomethacin for preterm labor: a randomized comparision of vaginal and rectal‐oral routes. Obstetrics & Gynecology 2000;95(4):482‐6. - PubMed
Bartfield 1998 {published data only}
-
- Bartfield M, Carlan SJ. The safety and efficacy of prolonged outpatient sulindac to prevent the recurrence of preterm labor: a prospective double‐blind study. Primary Care Update for Ob/Gyns 1998;5(4):178. - PubMed
Bivins 1993 {published data only}
-
- Bivins HA, Newman RB, Fyfe DA, Campbell BA, Stramm SL. Randomized comparative trial of indomethacin and terbutaline for the long term treatment of preterm labor. American Journal of Obstetrics and Gynecology 1993;168(1 Pt 2):375. - PubMed
-
- Bivins HA, Newman RB, Fyfe DA, Campbell BA, Stramm SL. Randomized trial of oral indomethacin and terbutaline sulfate for the long‐term suppression of preterm labor. American Journal of Obstetrics and Gynecology 1993;169:1065‐70. - PubMed
Caballero 1979 {published data only}
-
- Caballero A, Tejerina A, Dominguez A, Nava JM, Caballero AJ. Indomethacine alone or associated to ritodrine in the prevention of premature labour [abstract]. 9th World Congress of Gynecology and Obstetrics; 1979 Oct 26‐31;Tokyo, Japan. 1979:300.
Carlan 1992 {published data only}
-
- Carlan SJ, O'Brien WF, O'Leary TD, Mastrogiannis D. Randomized comparative trial of indomethacin and sulindac for the treatment of refactory preterm labor. Obstetrics & Gynecology 1992;79(2):223‐8. - PubMed
-
- Carlan SJ, O'Brien WF, O'Leary TD, Mastrogiannis DS. A randomized comparative trial of indomethacin and sulindac for the treatment of refactory preterm labor. American Journal of Obstetrics and Gynecology 1992;166:361. - PubMed
Carlan 1995 {published data only}
-
- Carlan S, Jones M, Schorr S, McNeill T, Rawji H, Clark K. Oral sulindac to prevent recurrence of preterm labor. American Journal of Obstetrics and Gynecology 1994;170:381. - PubMed
-
- Carlan SJ, O'Brien WF, Jones MH, O'Leary TD, Roth L. Outpatient oral sulindac to prevent recurrence of preterm labor. Obstetrics & Gynecology 1995;85:769‐74. - PubMed
-
- Jones M, Carlan S, Schorr S, McNeill T, Rawji H, Clark K, et al. Oral sulindac to prevent recurrence of preterm labor. American Journal of Obstetrics and Gynecology 1995;172:416. - PubMed
Ehsanipoor 2011 {published data only}
-
- Ehsanipoor RM, Shrivastava VK, Lee RM, Chan K, Galyean AM, Garite TJ, et al. A randomized, double‐masked trial of prophylactic indomethacin tocolysis versus placebo in women with premature rupture of membranes. American Journal of Perinatology 2011;28(6):473‐8. - PubMed
-
- Shrivastava V, Ehsanipoor R, Lee RM, Chan K, Gaylean A, Garite T, et al. Randomized double‐blinded trial of indomethacin tocolysis versus expectant management in patients with premature rupture of membranes at 24‐32 weeks of gestation. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S59.
Gamissans 1982 {published data only}
-
- Gamissans O, Canas E, Cararach V, Ribas J, Puerto B, Edo A. A study of indomethacin combined with ritodrine in threatened preterm labor. European Journal of Obstetrics & Gynecology and Reproductive Biology 1978;8:123‐8. - PubMed
-
- Gamissans O, Canas E, Cararach V, Ribas J, Puerto B, Edo A. A study of indomethacin combined with ritodrine in threatened preterm labor. Proceedings of 6th European Congress of Perinatal Medicine; 1978 Aug 29‐Sept 1 Vienna, Austria. 1978:Abstract no: 165. - PubMed
-
- Gamissans O, Cararach V, Serra J. The role of prostaglandin‐inhibitors, beta‐adrenergic drugs and glucocorticoids in the management of threatened preterm labor. In: Jung H, Lamberti G editor(s). Beta‐Mimetic Drugs in Obstetrics and Perinatology. Stuttgart: Georg Thieme, 1982:71‐84.
Gamissans 1984 {published data only}
-
- Gamissans O, Balasch J. Prostaglandin synthetase inhibitors in the treatment of preterm labor. In: Fuchs F, Stubblefield PG editor(s). Preterm Birth: Causes, Prevention and Management. New York: Macmillan, 1984:223‐48.
Groom 2005 {published data only}
-
- Groom KM, Shennan AH, Jones BA, Seed P, Bennett PR. Tocox‐‐a randomised, double‐blind, placebo‐controlled trial of rofecoxib (a cox‐2‐specific prostaglandin inhibitor) for the prevention of preterm delivery in women at high risk. BJOG: an international journal of obstetrics and gynaecology 2005;112:725‐30. - PubMed
Hallak 1992 {published data only}
-
- Hallak M, Moise KJ, Lira N, Dorman K, O'Brian Smith E, Cotton DB. The effect of tocolytic agents (indomethacin and terbutaline) on fetal breathing (FBM) and body movements (FM): a prospective, randomized, double blind, placebo‐controlled clinical trial. American Journal of Obstetrics and Gynecology 1992;166(1 Pt 2):375. - PubMed
-
- Hallak M, Moise KJ, Lira N, Dorman KF, O'Brian Smith E, Cotton DB. The effect of tocolytic agents (indomethacin and terbutaline) on fetal breathing and body movements: a prospective, randomized, double‐blind, placebo‐controlled clinical trial. American Journal of Obstetrics and Gynecology 1992;167(4):1059‐63. - PubMed
-
- Hallak M, Moise KJ, O'Brian Smith E, Cotton DB. The effects of indomethacin and terbutaline on human fetal umbilical artery velocimetry: a randomized double‐blind study. American Journal of Obstetrics and Gynecology 1993;168(1 Pt 2):348. - PubMed
-
- Hallak M, Moise KJ, O'Brian Smith E, Cotton DB. The effects of indomethacin and terbutaline on human fetal umbilical artery velocimetry: a randomized, double‐blind study. American Journal of Obstetrics and Gynecology 1993;168(3):865‐8. - PubMed
Humphrey 2001 {published data only}
-
- Humphrey RG, Bartfield MC, Carlan SJ, O'Brien WF, O'Leary TD, Triana T. Sulindac to prevent recurrent preterm labor: a randomized controlled trial. Obstetrics & Gynecology 2001;98(4):555‐62. - PubMed
Jain 2006 {published data only}
-
- Jain N, Gahlot D. A comparative study of isoxuprine versus ritodrine hydrochloride in the management of preterm labour [abstract]. 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6‐9; Cochin, Kerala State, India. 2006:148.
Katz 1983 {published data only}
-
- Katz Z, Lancet M, Yemini M, Mogilner BM, Feigl A, Ben‐Hur H. Treatment of premature labor contractions with combined ritodrine and indomethacine. International Journal of Gynecology & Obstetrics 1983;21:337‐42. - PubMed
Mital 1992 {published data only}
-
- Khuteta RP, Garg S, Bhargava A. Mefanimic acid in prevention of premature labour. 12th FIGO World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil. 1988:222.
-
- Mital P, Garg S, Khuteta RP, Khuteta S, Mital P. Mefenamic acid in prevention of premature labour. Journal of the Royal Society of Health 1992;112(5):214‐6. - PubMed
Newton 1991 {published data only}
-
- Newton ER, Shields L, Ridgway LE, Berkus MD, Elliott BD. Combination antibiotics and indomethacin in idiopathic preterm labor: a randomized double‐blind clinical trial. American Journal of Obstetrics and Gynecology 1991;165(6):1753‐9. - PubMed
-
- Ridgway L, Wright J, Newton E. The effect of indomethacin on fetal heart biometry. American Journal of Obstetrics and Gynecology 1991;164:349.
Rasanen 1995 {published data only}
-
- Jouppila P, Rasanen J. Response of the fetal hemodynamics to different maternal prostaglandin synthetase inhibitors. Proceedings of 14th European Congress of Perinatal Medicine; 1994 June 5‐8; Helsinki, Finland. 1994:Abstract no: 269.
-
- Jouppila P, Rasanen J. Response to the fetal hemodynamics to different maternal prostaglandin synthetase inhibitors. International Journal of Gynecology & Obstetrics 1994;46:48.
-
- Rasanen J, Jouppila P. Fetal cardiac function and ductus arteriosus during indomethacin and sulindac therapy for threatened preterm labor: a randomized study. American Journal of Obstetrics and Gynecology 1995;173(1):20‐5. - PubMed
Rios‐Anez 2001 {published data only}
-
- Rios‐Anez R, Santos‐Luque M, Noguera M. Use of an antagonist of the prostaglandins associated to a b‐sympathomimetic agent in the treatment of pre‐term labour. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 1):165.
Spearing 1979 {published data only}
-
- Spearing G. Alcohol, indomethacin and salbutamol. A comparative trial of their use in preterm labour. Obstetrics & Gynecology 1979;53:171‐4. - PubMed
Zuckerman 1984b {published data only}
-
- Zuckerman H, Shalev E, Gilad G, Katzuni E. Further study of the inhibition of premature labor by indomethacin Part I. Journal of Perinatal Medicine 1984;12:19‐23. - PubMed
References to studies awaiting assessment
Castillo 1988 {published data only}
-
- Castillo JM, Alonso J, Hernandez‐Garcia JM, Sancho B, Martinez V. Study of biochemical and biophysical modifications produced on pregnant women treated in threatened of premature labor with ritodrine and indometacine. 12th World Congress of Gynecology and Obstetrics; 1988 Oct 23‐28; Rio de Janeiro, Brazil. 1988:20.
Mesdaghinia 2012 {published data only}
-
- Mesdaghinia E, Mesdaghinia A, Hashemi T, Sooky Z, Mousavi GA. Comparing the effects fo indomethacin and magnesium‐sufate in the treatment of preterm labor. Feyz, Journal of Kashan University of Medical Sciences 2012;16(2):95‐101.
-
- Sooky Z. Comparison of delaying in premature labour by indomethacin and Mg sulphate in pregnant women referred to maternity hospital. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010) 2010.
Additional references
Babay 1998
-
- Babay Z, Lange IR, Amin H. Neonatal and maternal complications after administration of indomethacin for preterm labour between 24 and 30 weeks gestation. Journal of the Society of Obstetricians and Gynaecologists of Canada 1998;20(5):505‐11.
Crowther 2002
Doret 2002
-
- Doret M, Mellier G, Benchaib M, Piacenza JM, Gharib C, Pasquier JC. In vitro study of tocolytic effect of rofecoxib, a specific cyclo‐oxygenase 2 inhibitor. Comparison and combination with other tocolytic agents. BJOG: an international journal of obstetrics and gynaecology 2002;109:983‐8. - PubMed
Doyle 2009
Duckitt 2014
Eronen 1994
-
- Eronen M, Pesonen E, Kurki T, Teramo K, Ylikorkola O, Hallman M. Increased incidence of bronchopulmonary dysplasia after antenatal administration of indomethacin to prevent preterm labor. Journal of Pediatrics 1994;124(5):783‐8. - PubMed
FDA 2011
-
- U.S. Food, Drug Administration. Drug Safety Communication: New warnings against use of terbutaline to treat preterm labor. http://www.fda.gov/Drugs/DrugSafety/ucm243539.htm.
Flenady 2014a
Flenady 2014b
Gates 2004
-
- Gates S, Brocklehurst P. How should randomised trials including multiple pregnancies be analysed?. BJOG: an international journal of obstetrics and gynaecology 2004;111(3):213‐9. - PubMed
Gladstone 2011
Goldenberg 2008
Haas 2012
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Keirse 1992
-
- Keirse MJNC. Inhibitors of prostaglandin synthesis for treatment of preterm labour. In: Drife JO, Calder AA editor(s). Prostaglandins and the Uterus. London: Springer‐Verlag, 1992.
Koren 2006
-
- Koren G, Florescu A, Costei AM, Boskovic R, Moretti ME. Nonsteroidal antiinflammatory drugs during third trimester and the risk of premature closure of the ductus arteriosus: a meta‐analysis. Annals of Pharmacotherapy 2006;40(5):824‐9. - PubMed
McCain 1993
-
- McCain GC, Deatrick JA. The experience of high‐risk pregnancy. Journal of Obstetric, Gynecologic and Neonatal Nursing 1993;23:421‐7. - PubMed
Moise 1990
-
- Moise KJ, Ou C‐N, Kirshon B, Cano LE, Carpenter RJ. Placental transfer of indomethacin in the human pregnancy. American Journal of Obstetrics and Gynecology 1990;162:549‐54. - PubMed
Murray 2006
-
- Murray M. Antepartal and Intrapartal Fetal Monitoring. Springer Publishing Company, 2006.
Neilson 2014
Norman 2009
Norton 1993
-
- Norton ME, Merrill J, Cooper BA, Kuller JA, Clyman RI. Neonatal complications after the administration of indomethacin for preterm labor. New England Journal of Medicine 1993;329(22):1602‐7. - PubMed
Perron 2013
-
- Perron N, Tremblay E, Ferretti E, Babakissa C, Seidman EG, Levy E, et al. Deleterious effects of indomethacin in the mid‐gestation human intestine. Genomics 2013;101(3):171‐7. - PubMed
Petrou 2011
-
- Petrou S, Eddama O, Mangham L. A structured review of the recent literature on the economic consequences of preterm birth. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(3):F225–32. - PubMed
Powell 1995
-
- Powell SL, Holt VL, Hickok DE, Easterling T, Connell FA. Recent changes in delivery site of low‐birth‐weight infants in Washington: impact on birth weight‐specific mortality. American Journal of Obstetrics and Gynecology 1995;173(5):1585‐92. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2006
Sanchez‐Ramos 1999
-
- Sanchez‐Ramos L, Kaunitz AM, Gaudier FL, Delke I. Efficacy of maintenance therapy after acute tocolysis: a meta‐analysis. American Journal of Obstetrics and Gynecology 1999;181:484‐90. - PubMed
Slater 1999
-
- Slater DM, Dennes WJB, Campa JS, Poston L, Bennett PR. Expression of cyclo‐oxygenase types‐1 and ‐2 in human myometrium throughout pregnancy. Molecular Human Reproduction 1999;5(9):880‐4. - PubMed
Souter 1998
-
- Souter D, Harding J, McCowan L, O'Donnell C, McLeay E, Baxendale H. Antenatal indomethacin‐adverse fetal effects confirmed. Australian and New Zealand Journal of Obstetrics and Gynaecology 1998;38(1):11‐6. - PubMed
Su 2010
Van den Veyver 1993
-
- Veyver IB, Moise KJ. Prostaglandin synthetase inhibitors in pregnancy. Obstetrical & Gynecological Survey 1993;48(7):493‐502. - PubMed
Vogel 2014
Walker 2011
-
- Walker MW, Clark RH, Spitzer AR. Elevation in plasma creatinine and renal failure in premature neonates without major anomalies: terminology, occurrence and factors associated with increased risk. Journal of Perinatology 2011;31(3):199‐205. - PubMed
WHO 2012
-
- Howson CP, Kinney MV, Lawn JE. Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization, 2012.
Yelland 2011
-
- Yelland LN, Saltera AB, Ryana P, Makrides M. Analysis of binary outcomes from randomised trials including multiple births: when should clustering be taken into account?. Paediatric and Perinatal Epidemiology 2011;25:283–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

