Structural ensembles reveal intrinsic disorder for the multi-stimuli responsive bio-mimetic protein Rec1-resilin
- PMID: 26042819
- PMCID: PMC4455251
- DOI: 10.1038/srep10896
Structural ensembles reveal intrinsic disorder for the multi-stimuli responsive bio-mimetic protein Rec1-resilin
Abstract
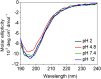
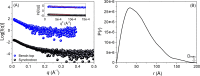
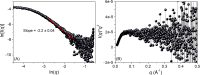
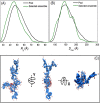
Rec1-resilin is the first recombinant resilin-mimetic protein polymer, synthesized from exon-1 of the Drosophila melanogaster gene CG15920 that has demonstrated unusual multi-stimuli responsiveness in aqueous solution. Crosslinked hydrogels of Rec1-resilin have also displayed remarkable mechanical properties including near-perfect rubber-like elasticity. The structural basis of these extraordinary properties is not clearly understood. Here we combine a computational and experimental investigation to examine structural ensembles of Rec1-resilin in aqueous solution. The structure of Rec1-resilin in aqueous solutions is investigated experimentally using circular dichroism (CD) spectroscopy and small angle X-ray scattering (SAXS). Both bench-top and synchrotron SAXS are employed to extract structural data sets of Rec1-resilin and to confirm their validity. Computational approaches have been applied to these experimental data sets in order to extract quantitative information about structural ensembles including radius of gyration, pair-distance distribution function, and the fractal dimension. The present work confirms that Rec1-resilin is an intrinsically disordered protein (IDP) that displays equilibrium structural qualities between those of a structured globular protein and a denatured protein. The ensemble optimization method (EOM) analysis reveals a single conformational population with partial compactness. This work provides new insight into the structural ensembles of Rec1-resilin in solution.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- DiMarco R. L. & Heilshorn S. C. Multifunctional materials through modular protein engineering. Adv. Mater. 24, 3923–3940 (2012). - PubMed
-
- Balu R., Whittaker J., Dutta N. K., Elvin C. M. & Choudhury N. R. Multi-responsive biomaterials and nanobioconjugates from resilin-like protein polymers. J. Mater. Chem. B. 2, 5936–5947 (2014). - PubMed
-
- Tamerler C. & Sarikaya M. Genetically designed peptide-based molecular materials. ACS Nano 3, 1606–1615 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

