Amyloid properties of the mouse egg zona pellucida
- PMID: 26043223
- PMCID: PMC4456372
- DOI: 10.1371/journal.pone.0129907
Amyloid properties of the mouse egg zona pellucida
Abstract
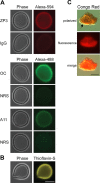
The zona pellucida (ZP) surrounding the oocyte is an extracellular fibrillar matrix that plays critical roles during fertilization including species-specific gamete recognition and protection from polyspermy. The mouse ZP is composed of three proteins, ZP1, ZP2, and ZP3, all of which have a ZP polymerization domain that directs protein fibril formation and assembly into the three-dimensional ZP matrix. Egg coats surrounding oocytes in nonmammalian vertebrates and in invertebrates are also fibrillar matrices and are composed of ZP domain-containing proteins suggesting the basic structure and function of the ZP/egg coat is highly conserved. However, sequence similarity between ZP domains is low across species and thus the mechanism for the conservation of ZP/egg coat structure and its function is not known. Using approaches classically used to identify amyloid including conformation-dependent antibodies and dyes, X-ray diffraction, and negative stain electron microscopy, our studies suggest the mouse ZP is a functional amyloid. Amyloids are cross-β sheet fibrillar structures that, while typically associated with neurodegenerative and prion diseases in mammals, can also carry out functional roles in normal cells without resulting pathology. An analysis of the ZP domain from mouse ZP3 and ZP3 homologs from five additional taxa using the algorithm AmylPred 2 to identify amyloidogenic sites, revealed in all taxa a remarkable conservation of regions that were predicted to form amyloid. This included a conserved amyloidogenic region that localized to a stretch of hydrophobic amino acids previously shown in mouse ZP3 to be essential for fibril assembly. Similarly, a domain in the yeast protein α-agglutinin/Sag 1p, that possesses ZP domain-like features and which is essential for mating, also had sites that were predicted to be amyloidogenic including a hydrophobic stretch that appeared analogous to the critical site in mouse ZP3. Together, these studies suggest that amyloidogenesis may be a conserved mechanism for ZP structure and function across billions of years of evolution.
Conflict of interest statement
Figures




References
-
- Litscher ES, Qi H, Wassarman PM. Mouse zona pellucida glycoproteins mZP2 and mZP3 undergo carboxy-terminal proteolytic processing in growing oocytes. Biochemistry 1999; 38:12280–12287. - PubMed
-
- Boja ES, Hoodbhoy T, Fales HM, Dean J. Structural characterization of native mouse zona pellucida proteins using mass spectrometry J Biol Chem. 2003; 278:34189–34202. - PubMed
-
- Greve JM, Wassarman PM. Mouse egg extracellular coat is a matrix of interconnected filaments possessing a structural repeat. J Mol Biol. 1985; 181:253–264. - PubMed
-
- Litscher ES, Janssen WG, Darie CC, Wassarman PM. Purified mouse egg zona pellucida glycoproteins polymerize into homomeric fibrils under non-denaturing conditions. J Cell Physiol. 2008; 214:153–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

