Paroxysmal nocturnal hemoglobinuria: a complement-mediated hemolytic anemia
- PMID: 26043387
- PMCID: PMC4695989
- DOI: 10.1016/j.hoc.2015.01.005
Paroxysmal nocturnal hemoglobinuria: a complement-mediated hemolytic anemia
Abstract
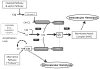
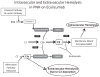
Paroxysmal nocturnal hemoglobinuria is manifests with a chronic hemolytic anemia from uncontrolled complement activation, a propensity for thrombosis and marrow failure. The hemolysis is largely mediated by the alternative pathway of complement. Clinical manifestations result from the lack of specific cell surface proteins, CD55 and CD59, on PNH cells. Complement inhibition by eculizumab leads to dramatic clinical improvement. While this therapeutic approach is effective, there is residual complement activity resulting from specific clinical scenarios as well as from upstream complement components that can account for suboptimal responses in some patients. Complement inhibition strategies are an area of active research.
Keywords: Alternative pathway of complement; Bone marrow failure; C1 inhibition; C3 blockade; Eculizumab; Hemolytic anemia; Humanized anti-C5 monoclonal antibody; Paroxysmal nocturnal hemoglobinuria.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
References
-
- Rosse WF. Paroxysmal nocturnal hemoglobinuria as a molecular disease. Medicine. 1997;76(2):63–93. - PubMed
-
- Brodsky RA. Narrative review: paroxysmal nocturnal hemoglobinuria: the physiology of complement-related hemolytic anemia. Ann Intern Med. 2008;148(8):587–95. - PubMed
-
- Miyata T, Yamada N, Iida Y, et al. Abnormalities of PIG-A transcripts in granulocytes from patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1994;330:249–55. - PubMed
-
- Rollins SA, Sims PJ. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J Immunol. 1990;144(9):3478–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

