Emerging roles of hypoxia-inducible factors and reactive oxygen species in cancer and pluripotent stem cells
- PMID: 26043406
- PMCID: PMC11916838
- DOI: 10.1016/j.kjms.2015.03.002
Emerging roles of hypoxia-inducible factors and reactive oxygen species in cancer and pluripotent stem cells
Abstract
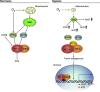
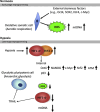
Eukaryotic organisms require oxygen homeostasis to maintain proper cellular function for survival. During conditions of low oxygen tension (hypoxia), cells activate the transcription of genes that induce an adaptive response, which supplies oxygen to tissues. Hypoxia and hypoxia-inducible factors (HIFs) may contribute to the maintenance of putative cancer stem cells, which can continue self-renewal indefinitely and express stemness genes in hypoxic stress environments (stem cell niches). Reactive oxygen species (ROS) have long been recognized as toxic by-products of aerobic metabolism that are harmful to living cells, leading to DNA damage, senescence, or cell death. HIFs may promote a cancer stem cell state, whereas the loss of HIFs induces the production of cellular ROS and activation of proteins p53 and p16(Ink4a), which lead to tumor cell death and senescence. ROS seem to inhibit HIF regulation in cancer cells. By contrast, controversial data have suggested that hypoxia increases the generation of ROS, which prevents hydroxylation of HIF proteins by inducing their transcription as negative feedback. Moreover, hypoxic conditions enhance the generation of induced pluripotent stem cells (iPSCs). During reprogramming of somatic cells into a PSC state, cells attain a metabolic state typically observed in embryonic stem cells (ESCs). ESCs and iPSCs share similar bioenergetic metabolisms, including decreased mitochondrial number and activity, and induced anaerobic glycolysis. This review discusses the current knowledge regarding the emerging roles of ROS homeostasis in cellular reprogramming and the implications of hypoxic regulation in cancer development.
Keywords: Cancer; Hypoxia-inducible factor; Reactive oxygen species; Stem cells.
Copyright © 2015. Published by Elsevier Taiwan.
Figures
References
-
- Mohyeldin A., Garzón‐Muvdi T., Quiñones‐Hinojosa A.. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010; 7: 150–161. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

