TCTEX1D2 mutations underlie Jeune asphyxiating thoracic dystrophy with impaired retrograde intraflagellar transport
- PMID: 26044572
- PMCID: PMC4468853
- DOI: 10.1038/ncomms8074
TCTEX1D2 mutations underlie Jeune asphyxiating thoracic dystrophy with impaired retrograde intraflagellar transport
Erratum in
-
Corrigendum: TCTEX1D2 mutations underlie Jeune asphyxiating thoracic dystrophy with impaired retrograde intraflagellar transport.Nat Commun. 2016 Mar 29;7:11270. doi: 10.1038/ncomms11270. Nat Commun. 2016. PMID: 27021811 Free PMC article. No abstract available.
Abstract
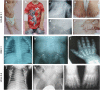
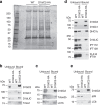
The analysis of individuals with ciliary chondrodysplasias can shed light on sensitive mechanisms controlling ciliogenesis and cell signalling that are essential to embryonic development and survival. Here we identify TCTEX1D2 mutations causing Jeune asphyxiating thoracic dystrophy with partially penetrant inheritance. Loss of TCTEX1D2 impairs retrograde intraflagellar transport (IFT) in humans and the protist Chlamydomonas, accompanied by destabilization of the retrograde IFT dynein motor. We thus define TCTEX1D2 as an integral component of the evolutionarily conserved retrograde IFT machinery. In complex with several IFT dynein light chains, it is required for correct vertebrate skeletal formation but may be functionally redundant under certain conditions.
Figures





References
-
- Fliegauf M., Benzing T. & Omran H. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell. Biol. 8, 880–893 (2007) . - PubMed
-
- Pedersen L.B. & Rosenbaum J.L. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr. Topics Dev. Biol. 85, 23–61 (2008) . - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- R01 DE019567/DE/NIDCR NIH HHS/United States
- P20 GM103449/GM/NIGMS NIH HHS/United States
- PG/07/045/22690/BHF_/British Heart Foundation/United Kingdom
- R37 GM030626/GM/NIGMS NIH HHS/United States
- T32 HG002536/HG/NHGRI NIH HHS/United States
- 098498/WT_/Wellcome Trust/United Kingdom
- RG/10/17/28553/BHF_/British Heart Foundation/United Kingdom
- 095515/WT_/Wellcome Trust/United Kingdom
- 100574/WT_/Wellcome Trust/United Kingdom
- R01 GM051293/GM/NIGMS NIH HHS/United States
- R01 AR066124/AR/NIAMS NIH HHS/United States
- RG/10/13/28570/BHF_/British Heart Foundation/United Kingdom
- R01 AR062651/AR/NIAMS NIH HHS/United States
- MR/L010305/1/MRC_/Medical Research Council/United Kingdom
- 100140/WT_/Wellcome Trust/United Kingdom
- G0800509/MRC_/Medical Research Council/United Kingdom
- 091551/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

