Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn's disease
- PMID: 26045134
- PMCID: PMC5136800
- DOI: 10.1136/gutjnl-2014-307649
Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn's disease
Abstract
Background: Crohn's disease (CD)-associated dysbiosis is characterised by a loss of Faecalibacterium prausnitzii, whose culture supernatant exerts an anti-inflammatory effect both in vitro and in vivo. However, the chemical nature of the anti-inflammatory compounds has not yet been determined.
Methods: Peptidomic analysis using mass spectrometry was applied to F. prausnitzii supernatant. Anti-inflammatory effects of identified peptides were tested in vitro directly on intestinal epithelial cell lines and on cell lines transfected with a plasmid construction coding for the candidate protein encompassing these peptides. In vivo, the cDNA of the candidate protein was delivered to the gut by recombinant lactic acid bacteria to prevent dinitrobenzene sulfonic acid (DNBS)-colitis in mice.
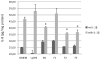
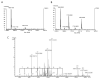
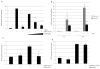
Results: The seven peptides, identified in the F. prausnitzii culture supernatants, derived from a single microbial anti-inflammatory molecule (MAM), a protein of 15 kDa, and comprising 53% of non-polar residues. This last feature prevented the direct characterisation of the putative anti-inflammatory activity of MAM-derived peptides. Transfection of MAM cDNA in epithelial cells led to a significant decrease in the activation of the nuclear factor (NF)-κB pathway with a dose-dependent effect. Finally, the use of a food-grade bacterium, Lactococcus lactis, delivering a plasmid encoding MAM was able to alleviate DNBS-induced colitis in mice.
Conclusions: A 15 kDa protein with anti-inflammatory properties is produced by F. prausnitzii, a commensal bacterium involved in CD pathogenesis. This protein is able to inhibit the NF-κB pathway in intestinal epithelial cells and to prevent colitis in an animal model.
Keywords: CELL BIOLOGY; CROHN'S DISEASE; IBD; INFLAMMATION; INTESTINAL BACTERIA.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures

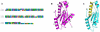



Comment in
-
The presence of the anti-inflammatory protein MAM, from Faecalibacterium prausnitzii, in the intestinal ecosystem.Gut. 2016 May;65(5):882. doi: 10.1136/gutjnl-2015-311094. Epub 2015 Dec 15. Gut. 2016. PMID: 26669616 No abstract available.
References
-
- MacDermott RP. Alterations of the mucosal immune system in inflammatory bowel disease. J Gastroenterol. 1996;31:907–16. - PubMed
-
- Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. - PubMed
-
- Allez M, Mayer L. Regulatory T cells: peace keepers in the gut. Inflamm Bowel Dis. 2004;10:666–76. - PubMed
-
- Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
