Metabolic reprogramming in macrophages and dendritic cells in innate immunity
- PMID: 26045163
- PMCID: PMC4493277
- DOI: 10.1038/cr.2015.68
Metabolic reprogramming in macrophages and dendritic cells in innate immunity
Abstract
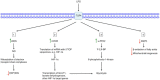
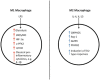
Activation of macrophages and dendritic cells (DCs) by pro-inflammatory stimuli causes them to undergo a metabolic switch towards glycolysis and away from oxidative phosphorylation (OXPHOS), similar to the Warburg effect in tumors. However, it is only recently that the mechanisms responsible for this metabolic reprogramming have been elucidated in more detail. The transcription factor hypoxia-inducible factor-1α (HIF-1α) plays an important role under conditions of both hypoxia and normoxia. The withdrawal of citrate from the tricarboxylic acid (TCA) cycle has been shown to be critical for lipid biosynthesis in both macrophages and DCs. Interference with this process actually abolishes the ability of DCs to activate T cells. Another TCA cycle intermediate, succinate, activates HIF-1α and promotes inflammatory gene expression. These new insights are providing us with a deeper understanding of the role of metabolic reprogramming in innate immunity.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

