Early performance of a miniaturized leadless cardiac pacemaker: the Micra Transcatheter Pacing Study
- PMID: 26045305
- PMCID: PMC4589655
- DOI: 10.1093/eurheartj/ehv214
Early performance of a miniaturized leadless cardiac pacemaker: the Micra Transcatheter Pacing Study
Abstract
Aims: Permanent cardiac pacing is the only effective treatment for symptomatic bradycardia, but complications associated with conventional transvenous pacing systems are commonly related to the pacing lead and pocket. We describe the early performance of a novel self-contained miniaturized pacemaker.
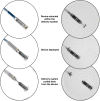
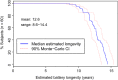
Methods and results: Patients having Class I or II indication for VVI pacing underwent implantation of a Micra transcatheter pacing system, from the femoral vein and fixated in the right ventricle using four protractible nitinol tines. Prespecified objectives were >85% freedom from unanticipated serious adverse device events (safety) and <2 V 3-month mean pacing capture threshold at 0.24 ms pulse width (efficacy). Patients were implanted (n = 140) from 23 centres in 11 countries (61% male, age 77.0 ± 10.2 years) for atrioventricular block (66%) or sinus node dysfunction (29%) indications. During mean follow-up of 1.9 ± 1.8 months, the safety endpoint was met with no unanticipated serious adverse device events. Thirty adverse events related to the system or procedure occurred, mostly due to transient dysrhythmias or femoral access complications. One pericardial effusion without tamponade occurred after 18 device deployments. In 60 patients followed to 3 months, mean pacing threshold was 0.51 ± 0.22 V, and no threshold was ≥2 V, meeting the efficacy endpoint (P < 0.001). Average R-wave was 16.1 ± 5.2 mV and impedance was 650.7 ± 130 ohms.
Conclusion: Early assessment shows the transcatheter pacemaker can safely and effectively be applied. Long-term safety and benefit of the pacemaker will further be evaluated in the trial.
Clinical trial registration: ClinicalTrials.gov ID NCT02004873.
Keywords: Leadless cardiac pacemaker; Miniaturization; Transcatheter pacing system.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures





Comment in
-
Leadless pacemakers: leading us into the future?Eur Heart J. 2015 Oct 1;36(37):2520-2. doi: 10.1093/eurheartj/ehv261. Epub 2015 Aug 16. Eur Heart J. 2015. PMID: 26282468 No abstract available.
References
-
- Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Kirchhof P, Blomstrom-Lundqvist C, Badano LP, Aliyev F, Bänsch D, Baumgartner H, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Hasdai D, Hoes AW, Le Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Tendera M, Van Gelder IC, Wilson CM; ESC Committee for Practice Guidelines (CPG), Document Reviewers. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013;34:2281–2329. - PubMed
-
- Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Tracy CM, Epstein AE, Darbar D, DiMarco JP, Dunbar SB, Estes NA, III, Ferguson TB, Jr, Hammill SC, Karasik PE, Link MS, Marine JE, Schoenfeld MH, Shanker AJ, Silka MJ, Stevenson LW, Stevenson WG, Varosy PD; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2013;61:e6–75. - PubMed
-
- Udo EO, Zuithoff NPA, van Hemel NM, de Cock CC, Hendriks T, Doevendans PA, Moons KG. Incidence and predictors of short- and long-term complications in pacemaker therapy: The FOLLOWPACE study. Heart Rhythm 2012;9:728–735. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

