Human oocytes. Error-prone chromosome-mediated spindle assembly favors chromosome segregation defects in human oocytes
- PMID: 26045437
- PMCID: PMC4477045
- DOI: 10.1126/science.aaa9529
Human oocytes. Error-prone chromosome-mediated spindle assembly favors chromosome segregation defects in human oocytes
Abstract
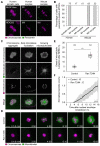
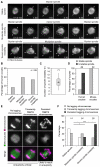
Aneuploidy in human eggs is the leading cause of pregnancy loss and several genetic disorders such as Down syndrome. Most aneuploidy results from chromosome segregation errors during the meiotic divisions of an oocyte, the egg's progenitor cell. The basis for particularly error-prone chromosome segregation in human oocytes is not known. We analyzed meiosis in more than 100 live human oocytes and identified an error-prone chromosome-mediated spindle assembly mechanism as a major contributor to chromosome segregation defects. Human oocytes assembled a meiotic spindle independently of either centrosomes or other microtubule organizing centers. Instead, spindle assembly was mediated by chromosomes and the small guanosine triphosphatase Ran in a process requiring ~16 hours. This unusually long spindle assembly period was marked by intrinsic spindle instability and abnormal kinetochore-microtubule attachments, which favor chromosome segregation errors and provide a possible explanation for high rates of aneuploidy in human eggs.
Copyright © 2015, American Association for the Advancement of Science.
Figures


References
-
- Pacchierotti F, Adler ID, Eichenlaub-Ritter U, Mailhes JB. Gender effects on the incidence of aneuploidy in mammalian germ cells. Environ Res. 2007;104:46–69. - PubMed
-
- Templado C, Vidal F, Estop A. Aneuploidy in human spermatozoa. Cytogenet Genome Res. 2011;133:91–99. - PubMed
-
- Danylevska A, Kovacovicova K, Awadova T, Anger M. The frequency of precocious segregation of sister chromatids in mouse female meiosis I is affected by genetic background. Chromosome Res. 2014;22:365–373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

