Cardiac hypertrophy is positively regulated by long non-coding RNA PVT1
- PMID: 26045764
- PMCID: PMC4440073
Cardiac hypertrophy is positively regulated by long non-coding RNA PVT1
Abstract
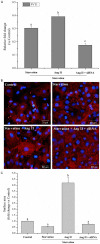
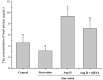
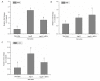
The aim of this study was to determine whether long non-coding RNA PVT1 can participate in the regulation of cardiac hypertrophy. A C57BL/6 mouse cardiac hypertrophic model was established using transverse aortic constriction (TAC). The animals subjected to sham operation were used as controls. Transcripts of PVT1 were analyzed in hearts of model and sham control groups after TAC for 4 weeks using quantitative real-time PCR (qRT-PCR). Additionally, to investigate whether PVT1 was involved in cardiac hypertrophy, 1 μM angiotensin II (Ang II) was used to induce hypertrophy and PVT1 siRNA was performed in the cultured neonatal mouse cardiac cardiomyocytes. Cell size was measured by cell surface area and total protein content analyses in response to Ang II treatment. Moreover, some hypertrophic markers including atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP), and beta-myosin heavy chain (β-MHC) were also quantified using qRT-PCR. As a result, PVT1 was up-regulated by 2.5-fold (P<0.05) in hypertrophic hearts after TAC for 4 weeks as compared to sham group. In addition, siRNA of endogenous PVT1 in cardiomyocytes significantly reduced (P<0.05) Ang II-induced increase of cell size in terms of cell surface area (by 5.6-fold) and total protein content (by 23.0%). PVT1 siRNA also obviously attenuated Ang II-induced ANP and β-MHC expression by 40.9% and 41.5%, respectively (P<0.05), but had no effect on BNP mRNA expression. Our results demonstrated that PVT1 was essential for the maintenance of cell size of cardiomyocytes and might play a role in the regulation of cardiac hypertrophy.
Keywords: Cardiac hypertrophy; PVT1; cardiomyocytes; long non-coding RNA; transverse aortic constriction.
Figures

References
-
- Song XW, Li Q, Lin L, Wang XC, Li DF, Wang GK, Ren AJ, Wang YR, Qin YW, Yuan WJ. MicroRNAs are dynamically regulated in hypertrophic hearts, and miR-199a is essential for the maintenance of cell size in cardiomyocytes. J Cell Physiol. 2010;225:437–443. - PubMed
-
- Jeong MH, Lee JS, Kim DH, Park WJ, Yang DK. Identification of novel microRNAs negatively regulating cardiac hypertrophy. Biochem Biophys Res Commun. 2012;428:191–196. - PubMed
-
- Li M, Wang N, Liu W, Zhi X, Zhang TC. Signaling Pathways in Cardiac Hypertrophy; Proceedings of the 2012 international conference on applied biotechnology (ICAB 2012); Springer; 2014.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
