Connections matter--how viruses use cell–cell adhesion components
- PMID: 26046138
- PMCID: PMC4311127
- DOI: 10.1242/jcs.159400
Connections matter--how viruses use cell–cell adhesion components
Abstract
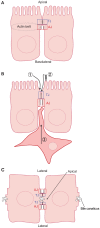
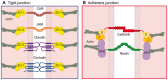
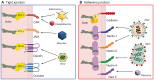
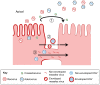
The epithelium is a highly organized type of animal tissue. Except for blood and lymph vessels, epithelial cells cover the body, line its cavities in single or stratified layers and support exchange between compartments. In addition, epithelia offer to the body a barrier to pathogen invasion. To transit through or to replicate in epithelia, viruses have to face several obstacles, starting from cilia and glycocalyx where they can be neutralized by secreted immunoglobulins. Tight junctions and adherens junctions also prevent viruses to cross the epithelial barrier. However, viruses have developed multiple strategies to blaze their path through the epithelium by utilizing components of cell–cell adhesion structures as receptors. In this Commentary, we discuss how viruses take advantage of the apical junction complex to spread. Whereas some viruses quickly disrupt epithelium integrity, others carefully preserve it and use cell adhesion proteins and their cytoskeletal connections to rapidly spread laterally. This is exemplified by the hidden transmission of enveloped viruses that use nectins as receptors. Finally, several viruses that replicate preferentially in cancer cells are currently used as experimental cancer therapeutics. Remarkably, these viruses use cell adhesion molecules as receptors, probably because--to reach tumors and metastases--ncolytic viruses must efficiently traverse or break epithelia.
Figures
References
-
- Andrus L., Marukian S., Jones C. T., Catanese M. T., Sheahan T. P., Schoggins J. W., Barry W. T., Dustin L. B., Trehan K., Ploss A. et al. (2011). Expression of paramyxovirus V proteins promotes replication and spread of hepatitis C virus in cultures of primary human fetal liver cells. Hepatology 54, 1901–1912 10.1002/hep.24557 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

