Motor and Nonmotor Circuitry Activation Induced by Subthalamic Nucleus Deep Brain Stimulation in Patients With Parkinson Disease: Intraoperative Functional Magnetic Resonance Imaging for Deep Brain Stimulation
- PMID: 26046412
- PMCID: PMC4469128
- DOI: 10.1016/j.mayocp.2015.03.022
Motor and Nonmotor Circuitry Activation Induced by Subthalamic Nucleus Deep Brain Stimulation in Patients With Parkinson Disease: Intraoperative Functional Magnetic Resonance Imaging for Deep Brain Stimulation
Abstract
Objective: To test the hypothesis suggested by previous studies that subthalamic nucleus (STN) deep brain stimulation (DBS) in patients with Parkinson disease would affect the activity of motor and nonmotor networks, we applied intraoperative functional magnetic resonance imaging (fMRI) to patients receiving DBS.
Patients and methods: Ten patients receiving STN DBS for Parkinson disease underwent intraoperative 1.5-T fMRI during high-frequency stimulation delivered via an external pulse generator. The study was conducted between January 1, 2013, and September 30, 2014.
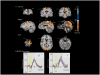
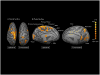
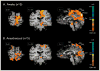
Results: We observed blood oxygen level-dependent (BOLD) signal changes (false discovery rate <0.001) in the motor circuitry (including the primary motor, premotor, and supplementary motor cortices; thalamus; pedunculopontine nucleus; and cerebellum) and in the limbic circuitry (including the cingulate and insular cortices). Activation of the motor network was observed also after applying a Bonferroni correction (P<.001) to the data set, suggesting that across patients, BOLD changes in the motor circuitry are more consistent compared with those occurring in the nonmotor network.
Conclusion: These findings support the modulatory role of STN DBS on the activity of motor and nonmotor networks and suggest complex mechanisms as the basis of the efficacy of this treatment modality. Furthermore, these results suggest that across patients, BOLD changes in the motor circuitry are more consistent than those in the nonmotor network. With further studies combining the use of real-time intraoperative fMRI with clinical outcomes in patients treated with DBS, functional imaging techniques have the potential not only to elucidate the mechanisms of DBS functioning but also to guide and assist in the surgical treatment of patients affected by movement and neuropsychiatric disorders.
Trial registration: clinicaltrials.gov Identifier: NCT01809613.
Copyright © 2015 Mayo Foundation for Medical Education and Research. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. The Lancet. Neurology. 2009 Jan;8(1):67–81. - PubMed
-
- Starr PA, Vitek JL, Bakay RA. Deep brain stimulation for movement disorders. Neurosurgery clinics of North America. 1998 Apr;9(2):381–402. - PubMed
-
- Pereira EA, Green AL, Stacey RJ, Aziz TZ. Refractory epilepsy and deep brain stimulation. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2012 Jan;19(1):27–33. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

