BEX1 acts as a tumor suppressor in acute myeloid leukemia
- PMID: 26046670
- PMCID: PMC4673273
- DOI: 10.18632/oncotarget.4095
BEX1 acts as a tumor suppressor in acute myeloid leukemia
Abstract
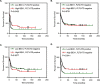
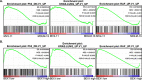
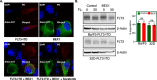
Acute myeloid leukemia (AML) is a heterogeneous disease of the myeloid lineage. About 35% of AML patients carry an oncogenic FLT3 mutant making FLT3 an attractive target for treatment of AML. Major problems in the development of FLT3 inhibitors include lack of specificity, poor response and development of a resistant phenotype upon treatment. Further understanding of FLT3 signaling and discovery of novel regulators will therefore help to determine additional pharmacological targets in FLT3-driven AML. In this report, we identified BEX1 as a novel regulator of oncogenic FLT3-ITD-driven AML. We showed that BEX1 expression was down-regulated in a group of AML patients carrying FLT3-ITD. Loss of BEX1 expression resulted in poor overall survival (hazard ratio, HR = 2.242, p = 0.0011). Overexpression of BEX1 in mouse pro-B and myeloid cells resulted in decreased FLT3-ITD-dependent cell proliferation, colony and tumor formation, and in increased apoptosis in vitro and in vivo. BEX1 localized to the cytosolic compartment of cells and significantly decreased FLT3-ITD-induced AKT phosphorylation without affecting ERK1/2 or STAT5 phosphorylation. Our data suggest that the loss of BEX1 expression in FLT3-ITD driven AML potentiates oncogenic signaling and leads to decreased overall survival of the patients.
Keywords: AKT; AML; FLT3; FLT3-ITD; apoptosis.
Conflict of interest statement
The authors declare no conflict of interests.
Figures


References
-
- Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532–1542. - PubMed
-
- Kabir NN, Rönnstrand L, Kazi JU. FLT3 mutations in patients with childhood acute lymphoblastic leukemia (ALL) Med Oncol. 2013;30:462. - PubMed
-
- Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, Löffler H, Sauerland CM, Serve H, Büchner T, Haferlach T, Hiddemann W. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. - PubMed
-
- Fröhling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, Döhner H, Döhner K leukemia AMLSGUAm. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100:4372–4380. - PubMed
-
- Kiyoi H, Yanada M, Ozekia K. Clinical significance of FLT3 in leukemia. International journal of hematology. 2005;82:85–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

